| Pages:
1
..
8
9
10
11
12
..
22 |
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Dead Woodchuck Update
Here is the first little gem:
Attachment: Rough_arc_prototype.mpeg (5.4MB)
This file has been downloaded 1195 times
I shifted away from the previous design that I posted. While the design worked at relatively low voltages, it took a lot of power to keep the arc
stable. Overall the design was clever, but fiddly. There is a reason why various arc processes came and went, but the Birkeland process hung around
for a few decades. It reaches a good balance between elegance, simplicity, and effectiveness.
This prototype uses 150V across the electrodes, as before, but includes an additional trigger coil to initiate the arc. A high voltage spark ionizes
the gap, allowing the lower voltage (at high current) to flow through it. A neodymium magnet placed underneath the arc blows it out almost
immediately, switching off the current until the gap is triggered again. High peak power can get dumped into the air gap (about 120W), but since the
arc is blown out quickly, the average power is quite low (about 3 to 5W). The average power can be adjusted by adjusting the frequency of the trigger
coil, as this controls how often the air gap fires. This enables the use of low-power parts. The electrodes themselves only get slightly warm during
operation.
In the video, it can be seen that the magnet blows the arc out into a semi-circular disk. Since the main current is DC, the magnetic force only
pushes the arc in one direction. If it was AC, a flat, round, flame disc would be seen between the electrodes.
It would be possible to replace the trigger coil and the high current power supply with a large neon sign transformer. There are some good reasons
why I did not do it that way. For one, they can be hard to find, and expensive. They are speciality items, and may not be available everywhere.
Second, it is easy to inadvertently kill oneself with one of those things. High voltage, high current, mmm...sizzle, sizzle, pop. I can smell
burning flesh from a dead body already. If high voltage was going to be used in the system, I didn't want it to be capable of sustaining a high
current arc by itself. I also didn't want to use a TV flyback transformer. Those are available, but are still speciality items, and are a bit
complicated to use if one is inexperienced.
I settled on the use of an automotive ignition coil to trigger the spark gap. These coils are available in every salvage yard, and probably even in
Walmart. The voltage is high enough from these things that it can reach out and grab you, but there isn't the high current capability that a neon
sign transformer has. I managed to get too close to the ignition coil (once), and receive a morning wakeup call. It was not pleasant, but it was
also not particularly dangerous.
This is intended to be a general status update. I haven't posted schematics or full details yet, and that probably won't happen until the final
report.
I also have a mostly completed prototype design, but I'm trying to figure out how to get the relevant videos to fit within the posting limits.
Hopefully I can post those shortly.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
This design uses the same electrodes as before, but they are now installed in a ceramic reaction chamber. A larger neodymium magnet is installed on
the bottom, affixed with epoxy. Later in the video, I show how the hoses are hooked between the arc chamber and a glass bottle. If one looks closely,
it is noticed that the jar contains NO2. A jar with clean air is placed next to it for comparison.
During the video, I turn the high voltage on and off, and also turn the high current supply on and off, just to show how it looks. During operation,
though, the arc is fairly stable. The trigger voltage is applied to the copper outlet tube in the center of the chamber. This arcs over internally
to the two electrodes, and provides reliable triggering.
I'm pushing air through the furnace with a small CPU fan mounted on the cardboard box. These fans don't like back pressure, and not much air is
moving through the column. However, considering that I'm only running about 5W into the chamber, the amount of airflow I have is probably about
right.
The parts are considerably oversized. I just used what I had laying around the lab. For a final go-around, I'll probably optimize the design to fit
into a small box.
There are three parts to the video, to keep file sizes manageable. I think I'll have to post them separately to get them to upload. If there is some
problem with the way I'm doing this, let me know and I'll figure something else out.
Right now, I am very tired, and have to go. I don't know how much sense I'm making right now. I may be occupied for the next few days as well.
Anyway, enjoy the update.
Attachment: update_01.mpeg (9.2MB)
This file has been downloaded 1036 times
Attachment: update_02.mpeg (9.4MB)
This file has been downloaded 929 times
Attachment: update_03.mpeg (9.2MB)
This file has been downloaded 945 times
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  |
I'm pushing air through the furnace with a small CPU fan mounted on the cardboard box. These fans don't like back pressure, and not much air is
moving through the column. However, considering that I'm only running about 5W into the chamber, the amount of airflow I have is probably about
right.
|
what about using a leaf blower or a hair dryer ?
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I haven't viewed the video clips yet. (They do not seem to be downloading properly for me.) But I would guess at this scale even a hair drier might
be too vigorous. Spark stability is likely to be affected if the air flow is too turbulent.
On a different note, I came home today with 3.5kg of soy beans given to me for free by a stock-feed company. They were kind enough to look up the
chemical tests for the batch of beans. 10.7% moisture and a whopping 43% protein. I am just about to soak them in some catalyst.

[Edited on 20-2-2015 by j_sum1]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
NICE! It don't come no cheaper than free  Excited to see the outcome
j_sum1! Excited to see the outcome
j_sum1!
What are you planning to use for your soak catalyst, KMnO4 solution or is it a trade secret 
Please post a pic of the cat soaked beans post drying, would love to see them.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I just put them in a bucket.
I based my mix on the 60:40 MnO2 CuO in the Charles Snowden Piggot paper. I had 50g of KMnO4 on hand and used 57.5g of copper sulfate pentahydrate
which I assume will decompose down to MnO2 and CuO at the right ratios. That is all a bit of a gamble, but it can't hurt. 1.7kg soaking as we speak
and it looks very purple.
The plan is to then dry them out with the catalyst diffused right through. Normally we would have 34°C days at this time of year but right now a
cyclone is passing through a couple of hundred km north and it is raining steadily with temps in the low 20s. I won't be able to dry under the tin
shed roof as planned and so they might have to go on the barbecue.
And I was very pleased with the price! I had spent a few hours scouting around a few local supermarkets with no success. One email to a stock feed
company and I had my offer within 20 minutes. All I needed to do was pick them up.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
J_sum1:
They're obviously expecting a massive rise in sales due to an anticipated boom in homemade nitrates, from soy beans!
[takes tongue out of cheek]
[Edited on 20-2-2015 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by j_sum1  | I just put them in a bucket.
I based my mix on the 60:40 MnO2 CuO in the Charles Snowden Piggot paper. I had 50g of KMnO4 on hand and used 57.5g of copper sulfate pentahydrate
which I assume will decompose down to MnO2 and CuO at the right ratios. That is all a bit of a gamble, but it can't hurt. 1.7kg soaking as we speak
and it looks very purple.
The plan is to then dry them out with the catalyst diffused right through. Normally we would have 34°C days at this time of year but right now a
cyclone is passing through a couple of hundred km north and it is raining steadily with temps in the low 20s. I won't be able to dry under the tin
shed roof as planned and so they might have to go on the barbecue.
And I was very pleased with the price! I had spent a few hours scouting around a few local supermarkets with no success. One email to a stock feed
company and I had my offer within 20 minutes. All I needed to do was pick them up. |
Your catalyst recipe sounds great and I'm happy you've included the copper. Such a pity about the weather 
Be careful if using heat to dry it... you might cook the beans in which case you could get a mess. At least the copper and manganese should keep the
soggy beans from rotting or germinating, hopefully 
You know, I've gotten so much by way of donations from companies towards my experimenting. The key is to design ideas around readily available
low-cost mass commodity materials and then usually they are quite happy to give you some.
Blogfast, the headline shall read "Vegan dynamite!".
"Explosive gas from beans"... how could we not have thought about it sooner, seems so obvious 
[Edited on 20-2-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by j_sum1  |
On a different note, I came home today with 3.5kg of soy beans given to me for free by a stock-feed company. They were kind enough to look up the
chemical tests for the batch of beans. 10.7% moisture and a whopping 43% protein. I am just about to soak them in some catalyst.
[Edited on 20-2-2015 by j_sum1] |
That's a theoretical maximum yield of 0.3 kg HNO3/kg beans at 100% nitrogen selectivity, but again, my gut feeling tells me getting 1/10 of that will
be a job well done!
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Quote: Originally posted by WGTR  |
This prototype uses 150V across the electrodes, as before, but includes an additional trigger coil to initiate the arc. A high voltage spark ionizes
the gap, allowing the lower voltage (at high current) to flow through it. |
Did you come up with that idea yourself? That's pretty genius.
I can't believe I didn't think of that, it's so simple. I have a 15Kv PSU, with less than 0.001 amps, useless for nitrogen oxidation because of the
small current. I'll give this a try sometime! I won't try to win this challenge though, it's your idea. Do you know how many volts your HV PSU is?
15KV makes on a ~.25 inch spark.
Good luck.
I've got an idea for a simpler setup, just have the arc in a conical flask above a solution of water or dilute nitric acid, then have a little
bubblier setup to dissolve oxygen and nitrogen tetroxide in the water (you could probably even do without a bubblier, if the nitrogen oxide production
is slow. Or use a stir bar).
Something like this:
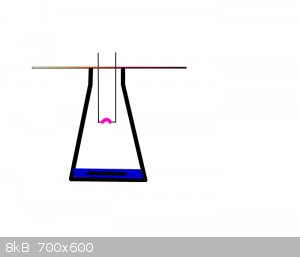
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | | That's a theoretical maximum yield of 0.3 kg HNO3/kg beans at 100% nitrogen selectivity, but again, my gut feeling tells me getting 1/10 of that will
be a job well done! |
I can't wait to see how this will pan out. 
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
My experience with such chemical innovations is that it's going to take a very great many attempts before getting things right. But's it's a bit of a
lottery, very rarely, you strike it lucky early on, though I must say, that has happened very seldom in my life. Your making of anhydrous AlCl3 was
one of those few concepts that worked relatively early on... very rare that that happens 
Actually this setup reminds me a lot of the 'billy moonshine stills. Ultimately, if proof of concept is established, I envision metal drums with ten's
of kg's of soy beans, fans bellowing air in, (a thumper  ) and absorbtion
column... all for a few jars of nitric acid hooch ) and absorbtion
column... all for a few jars of nitric acid hooch 
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by CuReUS  | Quote: Originally posted by WGTR  |
I'm pushing air through the furnace with a small CPU fan mounted on the cardboard box. These fans don't like back pressure, and not much air is
moving through the column. However, considering that I'm only running about 5W into the chamber, the amount of airflow I have is probably about
right.
|
what about using a leaf blower or a hair dryer ? |
That's a bit too much, I think. If too much air volume is used, the NO2 is diluted needlessly. The rate at which NO auto-oxidizes depends
on its concentration. Very high degrees of dilution in the gas mixture won't work very well in this process, I think. The air flow from the fan is
slow enough that I think I can, maybe, perhaps, feel a slight draft on the back of my hand at the outlet from the glass bottle. Then again, it could
be my imagination...even at this, in the video one can see that the bottle is filling with NO2. I have to go back later and calculate what
the air flow should be. In the commercial Birkeland Process, for a 0.5MW reactor only about 2m^3 was passed through the arc each minute.
Quote: Originally posted by Molecular Manipulations  | Quote: Originally posted by WGTR  |
This prototype uses 150V across the electrodes, as before, but includes an additional trigger coil to initiate the arc. A high voltage spark ionizes
the gap, allowing the lower voltage (at high current) to flow through it. |
Did you come up with that idea yourself? That's pretty genius.
I can't believe I didn't think of that, it's so simple. I have a 15Kv PSU, with less than 0.001 amps, useless for nitrogen oxidation because of the
small current. I'll give this a try sometime! I won't try to win this challenge though, it's your idea. Do you know how many volts your HV PSU is?
15KV makes on a ~.25 inch spark.
Good luck.
I've got an idea for a simpler setup, just have the arc in a conical flask above a solution of water or dilute nitric acid, then have a little
bubblier setup to dissolve oxygen and nitrogen tetroxide in the water (you could probably even do without a bubblier, if the nitrogen oxide production
is slow. Or use a stir bar).
Something like this: |
Thanks for the compliment. Yes, I came up with that myself. That's not to say that I'm the first person to do it, just that I figured it out in the
lab.
The way the air gap is triggered is not very obvious. One could look at the design, and think that it couldn't possibly work. After all, when the
high voltage gap is broken down (from the ignition coil), why does it also jump the secondary gap (low voltage, high current) instead of shorting out
through the 150V power supply? The reason is, that when the high voltage gap finally breaks down, it does so very suddenly, even explosively. The
voltage rises on the low voltage electrode within nanoseconds. At this rate, the inductance of the lead wires becomes very significant, and blocks the
high voltage long enough to force it across the secondary gap, triggering it.
The high voltage gap distance needs to be adjusted carefully, as the arc must decay completely between each high voltage trigger pulse. If it becomes
a continuous arc, then the secondary gap will not get triggered, as the 150V supply will short out the high voltage discharge. I'll explain all of
this more thoroughly later on.
I think the voltage output is something like 30-50kV. This is an automotive ignition coil (MSD Blaster 2, PN 8202) that is used to fire
spark plugs. The main difference between an automotive system and mine, is that instead of points I'm using a MOSFET (IRF740S), driving the gate with
a function generator. The condenser is placed in the usual location, across the MOSFET's D-S. The supply voltage is about 6V at 2-3A. A complete
automotive electronic ignition system could probably be jury-rigged to give the exact same results.
I don't mind at all if you want to try this yourself. I can't accept prize money for this anyway, so that's not an issue for me. I would certainly
be happy to see someone else interested in this.
Your idea is an interesting one, but may have one difficulty. If the sparking system is placed in a closed flask with water vapors, then the
electrodes, etc., will be exposed continually to nitric acid vapors. Lifetime of the system would probably be short. The outlet of my reactor uses a
copper tube. This is exposed to product gasses right as they come out of the reactor, as well as water vapor from the air. The key thing is that it
takes time for the NO produced to oxidize to NO2, and it takes time for this in turn to react with water. By the time any acid can be
formed, the gasses have already left the reactor, and are in a glass jar.
For the absorption column, I'm thinking of using a 3' glass tube, and packing it full of broken glass. This tube can be laid down on its side, and
filled halfway with water. This way, the gas mixture has to travel the full length of the tube, but doesn't need to bubble through water. Hopefully,
the glass chips will break up any laminar gas flow, and force the NO2 into contact with the water.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Beans, Gas and Sparks. Hmm.
Be careful out there !
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
A quick report on the beans.
Soaking overnight in my brew and nearly all the moisture was absorbed. Solution changed from purple to brown indicating a change of oxidation state
for the Mn. The beans now kill cockroaches. I saw one that tried to eat them.
It appears that the Mn and Cu have not actually soaked into the beans. The beans have swelled but the interior is not discoloured in any way. It all
seems to have been absorbed into the husk of the beans. I am going to blame cellular membranes that are designed to keep the nasties out of the
cells.
Now I need to carefully dry the beans out so that they burn. And the catalysts are likely contained only at the surface.
Next batch I will likely just give the dry beans a bit of a dusting in MnO2 and CuO. Maybe tumble them for a bit. That will save on the drying
process and likely give the same result in terms of the distribution of the catalyst.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Creating something that kills cockroaches is an impressive feat all by itself.
As an interesting idea for someone who wants to try it, here are some things that I came across:
Attachment: 11-7-13-10-20_university-of-illinois--nitrates-in-livestock-feed.pdf (101kB)
This file has been downloaded 586 times
Take their suggestions for minimizing nitrate production, and do the opposite. There is even a nitrate test specified for roughly determining
concentration.
The basic idea is that there is already nitrate in the soil, whether it originated from urea, ammonia, nitrate, etc. The plants just concentrate the
converted nitrate, and use it to create amino acids, etc. in the leaves. If the plant is stressed, then nitrate builds up in the stalks.
Some more information:
http://www.ianrpubs.unl.edu/pages/publicationD.jsp?publicati...
http://www.yara.com/doc/33527_Nitrogen_transformations_in_th...
[Edited on 2-23-2015 by WGTR]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Amazing finds WGTR and j_sum1
A Nitric Acid Competition, and a Bug Exterminator discovered !
The sheer innovation and imagination you guys are demonstrating is what this is all about.
For those that don't know, bugs tend Not to eat things that kill them, much like us, and most living things.
Finding something they Will eat, yet kills them, is the entire Bug Poison Industry's secret.
Prize fund increases to 350 euros.
Edit:
Anyone Stressing Plants by shouting 'Harvester' or 'Sheep' at them in order to increase their nitrate levels will be disqualified.
[Edited on 23-2-2015 by aga]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
To be more accurate, I found a roach crawling off the beans and looking decidedly unwell. (I was going to say, "seedy" but some things just
shouldn't be said.) I used my size tens to put an end to the bug. If anyone is retelling this story, I put it out of its misery. The reality
however is that I prefer squished roaches to crawling roaches even if they are nearly expired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Have the beans dried up yet j_sum1?
Talking about toxicity, one would have to guard against pets and even people accidentally eating those beans. This can be an issue for example if
tossing them out in a bin.
Soy is probably used in pet food... I'd hazard a guess that these metal oxides don't taste too bad due to their insolubility. However, they might
dissolve back to metal salts at the low pH of gastric juices making it a 'Trojan horse' bait 
Please mark the catalyst containing beans as TOXIC and store appropriately out of reach of children, pets and disgruntled wives.
*******************************************
aga, your prize money is generous, thank you for this great support towards amateur experimentalism!
[Edited on 24-2-2015 by deltaH]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by deltaH  | Quote: Originally posted by j_sum1  | I just put them in a bucket.
I based my mix on the 60:40 MnO2 CuO in the Charles Snowden Piggot paper. I had 50g of KMnO4 on hand and used 57.5g of copper sulfate pentahydrate
which I assume will decompose down to MnO2 and CuO at the right ratios. That is all a bit of a gamble, but it can't hurt. 1.7kg soaking as we speak
and it looks very purple.
The plan is to then dry them out with the catalyst diffused right through. Normally we would have 34°C days at this time of year but right now a
cyclone is passing through a couple of hundred km north and it is raining steadily with temps in the low 20s. I won't be able to dry under the tin
shed roof as planned and so they might have to go on the barbecue.
And I was very pleased with the price! I had spent a few hours scouting around a few local supermarkets with no success. One email to a stock feed
company and I had my offer within 20 minutes. All I needed to do was pick them up. |
Your catalyst recipe sounds great and I'm happy you've included the copper. Such a pity about the weather 
Be careful if using heat to dry it... you might cook the beans in which case you could get a mess. At least the copper and manganese should keep the
soggy beans from rotting or germinating, hopefully 
You know, I've gotten so much by way of donations from companies towards my experimenting. The key is to design ideas around readily available
low-cost mass commodity materials and then usually they are quite happy to give you some.
Blogfast, the headline shall read "Vegan dynamite!".
"Explosive gas from beans"... how could we not have thought about it sooner, seems so obvious 
[Edited on 20-2-2015 by deltaH] |
Soooo , Ill know who to blame when I have to dip my egg roll in milk.
This thread is still amazing me. you might actually have an easy way to do this (albeit a tough battle) in the end.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by Zombie  |
Soooo , Ill know who to blame when I have to dip my egg roll in milk.
This thread is still amazing me. you might actually have an easy way to do this (albeit a tough battle) in the end. |
It would be the nitric acid equivalent of winning the lotto, probably with similar odds to boot  But still, if there is ANY chance this could work, considering the outcome, it's worth taking! But still, if there is ANY chance this could work, considering the outcome, it's worth taking!
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Ahh the beans.
What I failed to appreciate is that 1.7kg of soy beans soaked to several times their original size and spread out ion a single layer for drying covers
a lot of square metres.
I have had my cockroach incident. I believe the mechanism of roach distress is crystallisation of copper salts internally. That's gotta be pleasant!
Bt I am not going to do a whole lot more investigation of that. It is outside my field.
I have had problems with germination. Well, some of the seeds have gone green and split. And I don't think it is copper.
I have had problems with mould. The atmosphere has been a bit too damp for effective drying.
I put the beans in shallow boxes on the shed roof in the sun while I went to work yesterday. I can't do the same today -- rain is forecast. The next
step will be to dry on the barbecue on Friday evening. Saturday is my next attempt at constructing my equipment and maybe attempting a run.
I mentioned before that the KMnO4 and CuSO4 appears to have not soaked into the beans. Next attempt will be with dry beans tumbled with MnO2 and CuO
with maybe enough liquid to make them stick. I'll probably go for either methylated spirits or acetone. But I have another secret ingredient that
might be advantageous as well.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Why don't you use milled soybeans mixed with powdered catalyst, just out of curiosity? Seems like the simplest way to maximize contact.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Two reasons.
1. I need to use what I have available. It took me a while to locate some beans in the first place.
2. The intention is to combust in a furnace. Pellets are going to allow for better air circulation than a powder -- at least in a simple set-up.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
How about pressure cooking in the liquid?
Perhaps you could eventually reduce the heat, and evaporate off the water.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
| Pages:
1
..
8
9
10
11
12
..
22 |