| Pages:
1
2
3
4
..
48 |
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
I have to say I didn't try anything with lead.
I was preparing to do so, but then decided that, given the apparent lack of evidence of success by others who've tried it, and the hassle of dealing
with the toxicity of the stuff, I decided it's best to try MMO type anodes first; since they are not only commercially available at moderate cost, but
since they are supposed to hold up so well.
If the MMO anode doesn't live up to expectations, I'll probably pick up where I left off with the PbO2.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I tried a bar of lead, and posted about it. Basically, at low temperature, it passivates with PbCl2, which then slowly oxidizes to a flakey
PbO2/PbCl2 deal. In hot brine, it works pretty reasonably to corrode the lead to Pb(OH)2, as you would expect from the solubility of PbCl2.
Tim
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
This is getting to be a major PITA.
I thought it would be relatively easy to find an affordable MMO pool chlorinator anode. Not so, in my experience so far...shrug...
Part of the reason for getting one is to answer the question of just how well MMO anodes make ClO3 and ClO4, how well they hold up, and to document
the results.
In order to do that in the most meaningful way, I need to know as precisely as possible just what type of coating I'm using...and therein lies a big
problem.
Most of the relevant patents seem to have expired, and now it's a big free-for-all out there...everything's apparently a "trade secret".
There's a big bunch of OEM's making pool chlorinators, and perhaps an even bigger bunch of manufacturers making "generic" replacement cells for them.
So you have all kinds of different coatings, i.e., different combinations of PGMs and PGM oxides alloyed with "film forming metal" oxides, etc., and
different manufacturing processes used to apply the coatings.
Just for asking one of the OEMs about their coating, I've basically been accused of being an industrial spy...needless to say they gave me no
information.
Anyway, I was laboring under the impression that any black or nearly black coating implied at least a PGM oxide coating...but then I discovered
something called "black ruthenium" plating, which is black and which is ruthenium but which is apparently not an oxide, and at least one vendor
selling an anode with a black coating claims his coatings are plain ruthenium. Another claims to be using pure iridium. Unfortunately I could find no
useful information regarding "black ruthenium" or similar types of plating.
So now, rather than getting the cheapest anode or what may be the best type, i.e., single plate vs multiple plates vs mesh, it looks like I may go
with the one for which I get the most detailed and reliable information regarding the coating, to the extent that's even possible in the first place.
Update: Apparently all the generic replacement cell products at saltcells.com have ruthenium oxide type MMO coatings. So I am leaning once again
toward the generic "sal-chlor" anode.
[Edited on 13-1-2006 by jpsmith123]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Ordered An Anode Today
For the few that may be interested, today I ordered an anode (actually I ordered the anode, cathode and insulators - the whole cell minus the
housing). The price for the cell parts was about $130.00 AUD, and international shipping was $25.00 AUD; the total cost in U.S. dollars was about
$118.00.
The parts are for a generic Sal-Chlor 12" cell from the following vendor:
http://www.directpoolsupplies.com.au/prod646.htm
The cell has a titanium plate anode (hopefully with MMO coating) and a titanium mesh cathode.
I probably won't receive it for a few weeks, but once I get it running I'll report on how it performs.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
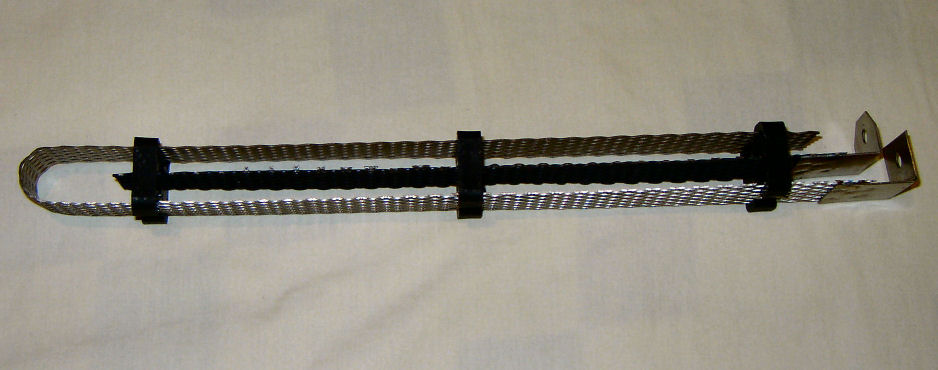
Finally Received The Chlorinator Cell Assembly
In case anyone's interested, the cell assy. arrived a few days ago.
I'll try to attach a picture of it.
Unfortunately the anode is not a solid plate, as I expected, but a "mesh plate". It should still work, but it will be more work to figure out exactly
what the working surface area is.
Both the MMO coated titanium mesh anode and the titanium mesh cathode are spot welded to titanium brackets that serve as electrical contacts.
The next problem is to find a suitable cell body, which will need to be at least about 14 inches deep.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Look what I found while searching for something different!
An article about the different types of Ti/noble metal anodes used in chlor-alkali and chlorate manufacture.
Note that they cannot be used to directly make perchlorates from chlorides.
To make perchlorates, chloride- free NaClO3 must be prepared first and this then electrolyzed in a separate cell.
http://docserver.ingentaconnect.com/deliver/cw/matthey/00321...
[Edited on 15-2-2006 by garage chemist]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
The link does not work for me garage chemist. Can you upload the paper?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
It doesn't work for me any more too... I'll try to fix it.
[Edited on 15-2-2006 by garage chemist]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Maybe you could split it into 2 pieces (if you have Adobe Acrobat)?
Or can you post a link to the site and I'll search for it? Thanks.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Here is a working link, click on the pdf button:
http://www.ingentaconnect.com/content/matthey/pmr/1998/00000...
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Thanks. Interesting historical perspective on the development of DSA anodes, but I didn't see anything about perchlorate production...only chlorate.
Did I miss something?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Those are the anodes that are used in pool chlorinators. I think you know them better than me since you surely did a lot of reading and literature
research before spending such an amount of money on one.
Anyway, perchlorates can also be conveniently made from chlorates by thermal decomposition, I've done it and the yields were rather good.
Best way is still electrolysis of chlorates with platinum anode. My platinum anode cost me only ca. 30€, since I made it myself from Pt wire (hint:
use 0,5mm diameter, they are the most economic. 0,3mm is too thin for most cells, although I had my first succes with it. It just takes longer since
the maximum current you can put through them is 2 A ).
[Edited on 16-2-2006 by garage chemist]
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Gouging rods
JP, the treatment for gouging rods involves peeling off the copper plating and then soaking
in linseed oil. This slows the erosion in the cell. I made 8 LBS of KClO4 using gouging rods.
It's a matter of technique. Keep the cell temperature below 40 C and the erosion proceeds
at a much slower rate. Use a cut up steel can for the cathode and don't worry about
the iron hydroxide that forms because the fluffy white shit is so insoluble that it, along with the
carbon bits filter out readily. I bend the steel to make an inverted V shape and the gouging
rod sits directly below it. This way, the rising chlorine runs into the hydroxide formed at the
cathode. My cell has a 5 litre volume.
[Edited on 17-2-2006 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
You mean those, right?
http://cgi.ebay.de/10-COPPER-CLAD-CARBON-CUTTING-RODS-NOS_W0...
They seem to be totally unknown in germany. I've never found any of them anywhere. I'd like to get some, since I could use them for maing bromates
(chlorates and perchlorates I already have enough).
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Gouging rods
Yes that's what I mean. Do you have a welding supply shop close to you ? That eBay price is
way too high ! I paid 26 USD for a box of 100 of these !
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Garage Chemist,
If I remember correctly, your perchlorate production started with NaClO3 (OTC as weed killer?); I'm wondering, did you do a batch type process, or did
you do a continuous process whereby you continually add chlorate and your NaClO4 precipitates out? And how did your Pt wire anode hold up?
I plan on first making chlorate, and then using that in a separate run to get perchlorate. (BTW it seems that in order to get maximum energy
efficiency and minimum anode wear (at least for platinum anodes) the best method, for either chlorate or perchlorate production, would be a
"continuous" process; see e.g., US Patent #5004527).
As of right now I will be using a pyrex 1L graduated cylinder as a cell body. I found an old 5 volt 15 amp power supply laying around that I hope will
suffice...maybe sometime next week I'll be able to start experimenting with it.
MadHatter,
I'm impressed that you were able to practically make useful amounts of KClO4 with graphite anodes. (I may try it one of these days as I found some
3/8" x 12" graphite rods lying around here - they're marked "National Intensiarc" - they look like gouging rods but they have no copper coating).
Anyway, I'm wondering, what kind of material did you use for your cell body?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
For cell body I used glass, a 250ml jar with screw-lid where the electrodes and gas exhaust were installed.
I used OTC (well, not in germany, but in France at that time- nowadays very hard to find even there) sodium chlorate solution (45% strength). It is
completely chloride- free as a test with AgNO3 solution showed, and this is also important for minimizing anode erosion.
2-4 g/L of potassium dichromate must be added to this in order to prevent reduction of chlorate at the cathode, which would produce chloride and cause
anode erosion.
The cell liquor must be magnetically stirred, as proper mixing is very important.
The power supply must be of the type with adjustable current limiting, otherwise you won't be able to keep a constant current.
And 15 A are WAY too much. For a 250ml cell, 2 A are good and 4 A the absolute maximum when used with a cooling bath.
A 0,5mm Pt wire is also not to be used with more than 4 A, or the solder connection will de-solder itself.
The maximum current that can be run through the cell depends firstly on the anode and secondly on the cooling and therefore on the surface of the cell
body that touches the cooling bath, and also on the thickness on the glass. Do not let the temperature rise above 40°C.
The progress can be nicely observed by watching the anode.
At the beginning, there will be only very slow oxygen evolution, as that oxygen goes into oxidation of the chlorate.
When there is only little chlorate left to oxidise, the oxygen production increases, and an ozone smell can be noticed, which gets more and more
intense as the chlorate is used up.
When nearly all chlorate is gone, there will be strong oxygen/ozone evolution and all energy now goes into splitting of the water.
I connect the exhaust of my cell (connection must be airtight, of course) to a wash bottle filled with water which serves as a bubble counter.
At the beginning, only hydrogen flows through it and the gas evolution is measured by counting the bubbles in a time span of, say, 30 seconds. When
the chlorate is used up, the gas evolution will have increased by 50% and the gas consists of a perfect hydrogen/oxygen mix which can be collected in
an inverted test tube and ignited FAR AWAY from the cell. It will explode with a loud bang, opposed to the very light puff that the pure hydrogen at
the beginning of the electrolysis makes.
To oxidise 1 mol of chlorate into perchlorate, 2 mol of electrons are needed. 1 mol of electrons is 26,8 Ah.
Calculate the required ampere hours and run at least the double amount of this through the cell.
When the cell is done, I have a ca. 50% NaClO4 solution (NaClO4 is very soluble and cannot be crystallized easily, so it is a batch- wise process).
I destroy residual chlorate by acidifying the solution, adding sodium disulfite and boiling. The solution must give off SO2, otherwise more
disulfite must be added. A SO2 smell from the solution is the sign that all chlorate has been reduced.
This also reduces the added dichromate to trivalent chromium, which is then precipitated as the hydroxide by adding NaOH solution and filtering.
From this I can make KClO4 by adding KCl solution (precipitate is very fine and difficult to filter and must always be recrystallized from boiling
water to make larger and purer crystals), or ammonium perchlorate (saturated NH4NO3 solution is needed for this, and NH4Cl is not soluble enough so
that the yields will be low if you use this). For ammonium perchlorate, strong cooling is necessary since it is quite soluble at ambient temperature.
Yield of KClO4 calculated from NaClO4 is often as high as 90- 95 %, even after recrystallization (at 0°C it is realy very sparingly soluble which
explains the good yields).
For ammonium perchlorate yields are about 50%.
A perchlorate cell is much cleaner to operate than a chlorate cell, but the parameters temperature, current density at the anode and composition of
the electrolyte must be closely controlled for it to work. If this is done, then a perchlorate cell is often much more efficient than a chlorate cell.
[Edited on 18-2-2006 by garage chemist]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: |
For cell body I used glass, a 250ml jar with screw-lid where the electrodes and gas exhaust were installed.
|
What was the lid made out of?
| Quote: |
2-4 g/L of potassium dichromate must be added to this in order to prevent reduction of chlorate at the cathode, which would produce chloride and cause
anode erosion.
|
I'm trying to get some persulfate for this purpose. If I can't find any I'll just have to get along without it. 
| Quote: |
The cell liquor must be magnetically stirred, as proper mixing is very important.
|
I'm hoping that, with the setup I have, the hydrogen evolution from the cathode will result in enough stirring.
| Quote: |
The power supply must be of the type with adjustable current limiting, otherwise you won't be able to keep a constant current.
|
For the way I plan on operating the cell, i.e., in a "continuous" manner, I'm thinking that the resistance won't fluctuate too drastically.
| Quote: |
And 15 A are WAY too much. For a 250ml cell, 2 A are good and 4 A the absolute maximum when used with a cooling bath.
|
Well 15 amps is the max output capacity of my power supply...I don't necessarily intend to run at that current.
| Quote: |
A 0,5mm Pt wire is also not to be used with more than 4 A, or the solder connection will de-solder itself.
|
For now I'll be using the MMO pool chlorinator anode, which supposedly can handle lots more than 4 Amps, but I plan on not being very aggressive, at
least until I get a feel for what's going on.
| Quote: |
The maximum current that can be run through the cell depends firstly on the anode and secondly on the cooling and therefore on the surface of the cell
body that touches the cooling bath, and also on the thickness on the glass. Do not let the temperature rise above 40°C.
|
I'll be using a 1 L pyrex graduated cylinder, which is relatively long and narrow and should therefore make for efficient cooling; i.e., hopefully the
radial temperature gradient will be small.
| Quote: |
The progress can be nicely observed by watching the anode.
At the beginning, there will be only very slow oxygen evolution, as that oxygen goes into oxidation of the chlorate.
When there is only little chlorate left to oxidise, the oxygen production increases, and an ozone smell can be noticed, which gets more and more
intense as the chlorate is used up.
When nearly all chlorate is gone, there will be strong oxygen/ozone evolution and all energy now goes into splitting of the water.
I connect the exhaust of my cell (connection must be airtight, of course) to a wash bottle filled with water which serves as a bubble counter.
At the beginning, only hydrogen flows through it and the gas evolution is measured by counting the bubbles in a time span of, say, 30 seconds. When
the chlorate is used up, the gas evolution will have increased by 50% and the gas consists of a perfect hydrogen/oxygen mix which can be collected in
an inverted test tube and ignited FAR AWAY from the cell. It will explode with a loud bang, opposed to the very light puff that the pure hydrogen at
the beginning of the electrolysis makes.
|
Sounds like a clever idea...watching for increased gas evolution that way...to determine when to harvest the ClO4.
Some people claim that they wait until they smell ozone, but if I were to do a batch process, I think I'd rather have a visual indication.
| Quote: |
When the cell is done, I have a ca. 50% NaClO4 solution (NaClO4 is very soluble and cannot be crystallized easily, so it is a batch- wise process).
|
I'm hoping that by continually adding NaClO3, the NaClO4 will precipitate out.
| Quote: |
I destroy residual chlorate by acidifying the solution, adding sodium disulfite and boiling. The solution must give off SO2, otherwise more
disulfite must be added. A SO2 smell from the solution is the sign that all chlorate has been reduced.
|
I'm hoping that a continuous process will lead to a fairly pure product, but I have a supply of Na2S2O5, so that's what I'll do if need be.
| Quote: |
From this I can make KClO4 by adding KCl solution (precipitate is very fine and difficult to filter and must always be recrystallized from boiling
water to make larger and purer crystals), or ammonium perchlorate (saturated NH4NO3 solution is needed for this, and NH4Cl is not soluble enough so
that the yields will be low if you use this). For ammonium perchlorate, strong cooling is necessary since it is quite soluble at ambient temperature.
|
In my case, I want at least some of the perchlorate as NaClO4. Have you ever recovered and kept any solid NaClO4, or did you immediately go from
liquor to KClO4?
BTW, I'm curious, how well is your Pt anode wire holding up?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The lid was the ordinary plastic coated metal lid it came with (it originally contained tomato sauce  ). ).
The connections and exhaust were fitted into the lid by drilling holes and using silicone. This worked well.
Make yourself some potassium dichromate, it's very easily made from Cr2O3 (available at art supply stores). Go to frogfot's site for a detailed
synthesis.
And the hydrogen evolved will NOT result in satisfactory stirring. Use a magnetic stirrer, it can be as simple as a magnet on a motor under the cell
and a steel bar coated with plastic or sealed into a glass ampoule inside it.
Otherwise acidic layers will develop around the anode and attack it.
You cannot precipitate NaClO4 by adding anything, it is far more soluble than NaClO3! Even boiling down the cell liquor to half its volume and
cooling won't make any solid NaClO4.
I use my NaClO4 as the 50% aqueous solution I get by destroying the chlorate and precipitating the chromium, the only impurities are sodium sulfate,
sulfite and chloride when HCl is used for acidification.
For all reactions using it it has to be dissolved anyway, so it would be a waste of time to isolate the solid salt, which is a real pain. I speak from
experience (boiling down the solution will make a thick sludge from which no crystals can be obtained).
NaClO4 cannot be obtained in a continous process, as it can't be separated from the solution.
It is produced as the 50% solution in water which can be used for all desired purposes.
If the cell is operated correctly, the Pt wire is not visibly attacked.
The only time it got attacked was when I ran the cell without dichromate.
After this, the cell also contained some chloride due to reduction of chlorate at the cathode.
Persulfate will not prevent this. For platinum anode, dichromate has to be used.
It forms a diaphragm of hydrous chromium oxide around the cathode, preventing the contact of nascent hydrogen with the electrolyte.
[Edited on 19-2-2006 by garage chemist]
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Perchlorate
1st, I need to correct an error I made on the gouging rods:
That was 26 USD for 50 - not 100. My apologies. Still thats only 52 cents a rod.
My cell body is basically a 5 litre glass cookie jar that I found at Walmart. I like the V-shape
cathode because the chlorine or oxygen rises from the anode to mix with cathode
products. This is why I don't need to stir. The worst possible configuration for the
electrodes is to have them sitting on opposite sides of the cell. Very little mixing occurs.
As mentioned before, keeping the cell temperature below 40C(104F) keeps the rate of
erosion down.
Solubilities for compounds from CRC 62nd Edition(1981-1982), grams per 100ml H2O:
KClO4 .75 @ 0C 21.8 @ 100C
NaClO4.H2O 209.0 @ 15C 284.0 @ 50C
I start my electolyte at 9% by weight KCl. A little NaCl is mixed in because oxidation
of NaCl is more efficient than KCl. The metathesis reaction with KCl forces KClO4 out of
solution because of its much lower solubility. I put 2g of NaF in the electolyte to improve
current efficiency. Voltage is at 6 and amps are adjusted to keep the cell temperature
below 40C. After completion, FeSO4 is used to destroy any residual chlorate. The rest is
fractional crystallization to get the KClO4.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: |
The lid was the ordinary plastic coated metal lid it came with (it originally contained tomato sauce  ). ).
The connections and exhaust were fitted into the lid by drilling holes and using silicone. This worked well.
|
I was wondering how well silicone adhesives hold up to the brine. I'm glad to hear it works.
| Quote: |
And the hydrogen evolved will NOT result in satisfactory stirring. Use a magnetic stirrer, it can be as simple as a magnet on a motor under the cell
and a steel bar coated with plastic or sealed into a glass ampoule inside it.
|
Since I have little relevant literature and I have yet to make any ClO4 myself, I can't really agree or disagree with you. I do know that other people
have made ClO4 without additional stirring...whether or not they paid a significant penalty in terms of low efficiency or anode wear, I don't know.
I wonder if air bubbled in from an aquarium air pump would help?
| Quote: |
You cannot precipitate NaClO4 by adding anything, it is far more soluble than NaClO3! Even boiling down the cell liquor to half its volume and
cooling won't make any solid NaClO4.
|
Since the solubility of NaClO4 although relatively high is nevertheless finite, I would guess that getting it out of solution is possible.
| Quote: |
I use my NaClO4 as the 50% aqueous solution I get by destroying the chlorate and precipitating the chromium, the only impurities are sodium sulfate,
sulfite and chloride when HCl is used for acidification.
For all reactions using it it has to be dissolved anyway, so it would be a waste of time to isolate the solid salt, which is a real pain. I speak from
experience (boiling down the solution will make a thick sludge from which no crystals can be obtained).
NaClO4 cannot be obtained in a continous process, as it can't be separated from the solution.
It is produced as the 50% solution in water which can be used for all desired purposes.
|
I almost hate to speak on the issue, since I've yet to try it myself, but others are claiming it's possible. One person who's made a few posts here,
"gilbert pinkston", has claimed he got NaClO4 crystals directly from his electrolysis cell...and he's provided pictures, which I will try to attach.
| Quote: |
If the cell is operated correctly, the Pt wire is not visibly attacked. The only time it got attacked was when I ran the cell without dichromate.
After this, the cell also contained some chloride due to reduction of chlorate at the anode.
|
I think you meant to say that the reduction would occur at the cathode?
| Quote: |
Persulfate will not prevent this.
|
You may be correct, however it is purported to enhance efficiency, and I assumed the mechanism would be by way of prevention of the reduction of
chlorate.
[Edited on 29-10-2008 by chemoleo- resized pic]
[Edited on 29-10-2008 by chemoleo]
 
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Of course I meant reduction at the cathode. what a stupid typo!
I haven't treid persulfate, but I don't want to attack the platinum another time, so I keep on using dichromate from which I know it works.
That crystallized NaClO4 looks interesting. That solution must be at ca. 70% concentration for this to occur (solubility 200g/100ml water at 0°C).
And only a small portion of the NaClO4 will crystallize. The solubility is really extremely high!
For a continuos process, you can elctrolyze a KClO3 solution (easier to make and purify than NaClO3 due to stronger temperature dependence of
solubility) at 0°C.
The formed KClO4 will directly crystallize at the anode and fall down to the bottom. After no more KClO4 is formed, the solution can simply be
filtered or decanted and re-saturated with KClO3.
An improvement of this process would be to hang a cotton bag filled with solid KClO3 into the elctrolyte to replenish KClO3 in the elctrolyte at the
same rate as it is used up. This would need some stirring, of course.
The obvious disadvantage of this process is that only KClO4 can be made like this.
Stirring with air blown in by a pump would be a good idea, I think this would suffice for efficient stirring in a perchlorate cell. This technique is
known as air stirring and is used for the stirring of nitroglycerine batches (to minimize mechanical shock).
It would be a good substitute for magnetic stirring.
Of course this means that you can't measure the gas evolution any more, but it isn't really important.
MadHatter, did you make it from chloride directly to perchlorate with only graphite? That's very remarkable. The low temperature and low current
density in your cell has apparently largely prevented erosion of the graphite.
But the current efficiency must have been really low, since chlorate cells need high temperature for high efficiency (but high temperature also favors
erosion of the graphite...).
But they work with low temperature, too. The chlorine dissolves even better at low temp, so the pH would have to be adjusted less often than with a
hot cell.
[Edited on 19-2-2006 by garage chemist]
[Edited on 19-2-2006 by garage chemist]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: |
That crystallized NaClO4 looks interesting. That solution must be at ca. 70% concentration for this to occur (solubility 200g/100ml water at 0°C).
And only a small portion of the NaClO4 will crystallize. The solubility is really extremely high!
|
Well I don't know exactly how he operates his cell, but my guess is that he starts with highly concentrated NaClO3 solution, maybe he adds some
additional NaClO3 to the cell as the electrolysis proceeds, in order to build up a highly concentrated NaClO4 solution, and then at some point he
turns off the power and adds more NaClO3, which, by way of the common ion effect, will cause an appreciable amount of the NaClO4 to precipitate out.
And then the cell is already prepared for another run.
| Quote: |
For a continuos process, you can elctrolyze a KClO3 solution (easier to make and purify than NaClO3 due to stronger temperature dependence of
solubility) at 0°C.
The formed KClO4 will directly crystallize at the anode and fall down to the bottom. After no more KClO4 is formed, the solution can simply be
filtered or decanted and re-saturated with KClO3.
An improvement of this process would be to hang a cotton bag filled with solid KClO3 into the elctrolyte to replenish KClO3 in the elctrolyte at the
same rate as it is used up. This would need some stirring, of course.
The obvious disadvantage of this process is that only KClO4 can be made like this.
|
I was considering some kind of bag to hold the chlorate (or chloride, as the case may be). I was thinking maybe some kind of PVC mesh. Will cotton
hold up?
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
KClO4
Garage Chemist, that's one tip I hadn't thought about - using an air pump(aquarium)
to achieve the stirring effect. Easier to setup than a magnetic stirrer. Thanks !
Websites that can help:
Wouter: http://www.wfvisser.dds.nl/EN/kclox_EN.html
Cape Canaveral: http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
Perchlorate production begins when the chloride(by weight) drops below 10%. This is why I
start my electrolyte at 9% to get things going immediately. I have to replace the gouging rods
a few times during the process but that's fine with me because they're cheap to use.
8 LBS of KClO4 for about 3.12 USD(6 rods) is bargain IMHO. I still have almost 90 rods left.
A few were consumed early on while tweaking the technique and of course producing
straight chlorate using saturated KCl solutions. The lovely cactus needle like formations
are indications that the chlorate is being produced.
Dichromates, fluorides, and persulphates are used to increase the efficiency of the operation.
I've used all 3 at different times. Personally, I like NaF. It works very well and it's cheap.
As for cathodic protection, I couldn't give a damn. I'm not worried about consuming a
soup can.
I haven't had much time lately to experiment with anodes but I do have the dioxides of lead,
manganese, and titanium to work with. I also have about 2 meters of titanium rod to use as
a substrate for coating lead dioxide(from the acetate plating bath) to work with. The problem
is getting a thick enough coating of PbO2 onto the Ti so it won't be attacked during the
electrolysis.
[Edited on 19-2-2006 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MadHatter
Garage Chemist, that's one tip I hadn't thought about - using an air pump(aquarium)
to achieve the stirring effect. Easier to setup than a magnetic stirrer. Thanks !
|
Hey, that was my idea! 
BTW MadHatter, do you remember what kind of anode current density you were running to make your KClO4?
|
|
|
| Pages:
1
2
3
4
..
48 |