vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
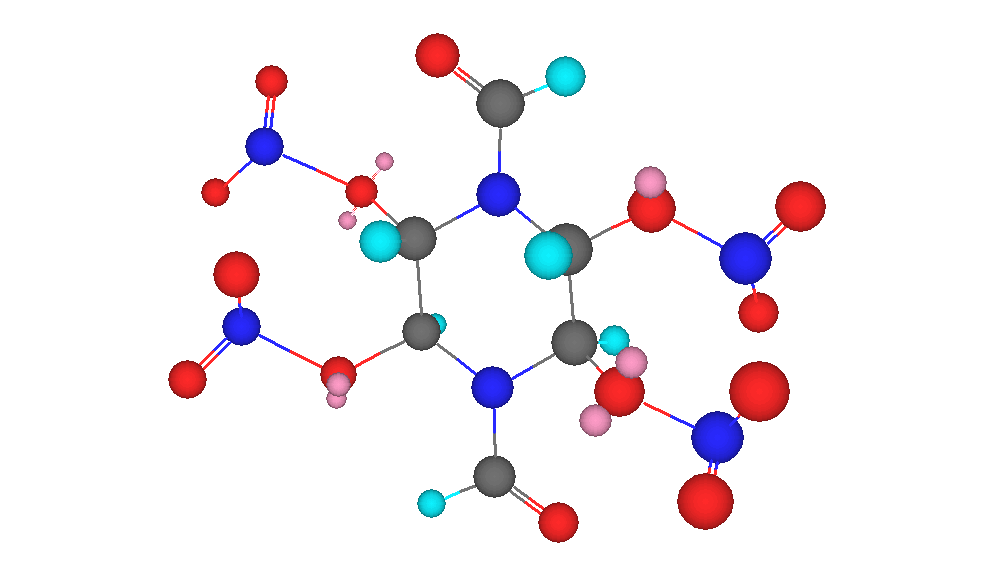
1,4-Diformyl-2,3,5,6-Tetranitropiperazine
1,4-Diformyl-2,3,5,6-Tetranitropiperazine is a new primary explosive based on glyoxal.
Firstly, 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine is synthesised by reacting 2 glyoxal ( HOC-COH ) molecules with 2 molecules of formamide (
COH-NH<sub>2</sub> ) in H<sub>2</sub>O at a pH of 9.
Then, 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine is nitrated by 100% HNO<sub>3</sub> with trifluoroaceticanhydride as the solvent.
All is carried out with an icebath and after the last step the solution is allowed to warm up to ambient temperature under stirring.
1,4-Diformyl-2,3,5,6-Tetranitropiperazine seems to form rather small crystals, as the authors of the article needed several recrystallisations to get
crystals that were suitable for X-ray diffraction.
1,4-Diformyl-2,3,5,6-Tetranitropiperazine is a primary which is sensitive to shock, but only deflagrates violently when heated.
What strikes me is the use of trifluoroaceticanhydride as a solvent for nitrating. Apart from using it to create
N<sub>2</sub>O<sub>5</sub> I wonder if they specifically use it to avoid any addition reactions on the ring structure?
Or maybe because it isn't flammable?
This information comes from the JoPEP nr1, 2003.
3D molecule was drawn by me using Chem3D Ultra.
To clarify, the little pink bubbles beside the oxygen atoms are lone electron pairs.
[Edited on 9-3-2003 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Hmm, Afraid to post because it sounds complicated? This should be a fairly easy compound to prepare, especially if you keep in mind that the TFAA and
100% nitric acid aren't really necessary. Remember, this comes from a published research article, which means the authors will use the most
sophisticated and highest yielding method since they're only analysing small amounts.
This is not a batch synthesis procedure.
C'mon guys...
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Hmm, not sure what to say about this, as I'm a bit of a novice in the area of Organic Chemistry and have little experience with Explosives  . .
I suppose one way to make Glyoxal could be to use some strong dessicant on Formic Acid... Vague, yes, but I can't think of specifics at the
moment...just laying the ground works for this  . .
As for Formamide... would reacting a Formyl Halide with Ammonia work to create Formamide?
COHCl + 2 NH3 --> COH-NH2 + NH4Cl
I have no idea about the Trifluoroacetic Anhydride, so I'm not going to say anything about it...to hopefully avoid making an ass of myself  . .
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
It seems that glyoxal is a very useful chemical to have around! This, TENGU, HNIW... loads of good things need it.
Formamide can be made by reacting formic acid with ammonia and then dehydrating it with something like P2O5 I would have thought, or maybe by heating
ammonium formate with H2SO4.
But unless anyone can find an alternative to TFAA, or a reference which says it isn't needed, then I'll stick to lead azide  . .
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
My "sources" say that glyoxal can be made by oxidizing ethanol or acetaldehyde (ethanal) with nitric acid.
I do not have any specific procedure though.
Mr Samose, I don't see how one could make glyoxal from formic acid, as glyoxal has 2 carbon atoms -> HOC-COH
Or do you mean a condensation reaction?
I think the TFAA could be substituted by something else. They probably used it to avoid any side reactions.
This is just the first reported preparation, so there's no doubt more simple methods will be discovered.
Then we might actually making some progress Mr Samosa!
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Actually, I was thinking of simply dehydrating Formic Acid to make "Formyl Anhydride," if that is the correct nomenclature for the
product...
But just now do I realize my mistake- this would not be the desired product! oops 
As for oxidizing Ethanol, do you suppose that reaction could work by creating Nitric Acid in situ using Sulfuric Acid and Potassium or Ammonium
Nitrate? If this is the case, then this would certainly be the easiest way to go about obtaining Glyoxal. The hell with Acetaldehyde, because not
everyone can get such things  . .
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Trifluoroacetic anhydride has a low bp and is easily removed as the acid; you thus need less heat to efficiently remove under vaccuum the solvant;
also with such sensitive HE primaries, it is a good idea to keep the stuff cool when isolating!
TFA acid is a very strong inoxydable acid!
What puzzles me is that there is no mention of nitrolysis of the formyl moiety!
The use of TFA acid anhydride seem not to be needed!As the same kind of reactions should occure well in 100% HNO3 or HNO3/acetic anhydride!
O=C-NH2 + O=CH-CH=O + NH2-CH=O --> O=CH-NH-CHOH-CHOH-NH-CH=O
O=CH-NH-CHOH-CHOH-NH-CH=O + O=CH-CH=O --> O=CH-N(CHOH-CHOH)2N-CH=O
Then reacting with HNO3 yields tetranitrate ester (you thus have a kind of dimeric EGDN and the hability of easy cristallisation owing to amide
moiety) --> suspected sensitivity equal to PETN/MHN and VOD close to that of MHN!Excess OB from the NO3 is compensated by the end O=CH-!
What would be much more interesting would be to do would be to do the same reaction after hydrolysis of the O=CH-N(CHOH-CHOH)2N-CH=O that should yield
HN(CHOH-CHOH)2NH that is one of the product of the condensation of glyoxal with NH3 (2mole/2 mole)!
Then action of HNO3 conc should yield:
1,4 dinitro-1,4 diaza-2,3,5,6 tetrol-cyclohexane tetranitrate O2N-N(CHONO2-CHONO2)2N-NO2
The condensation of glyoxal with NH3 yield (3moles/4moles):
CHOH -CHOH
NH NH
CH -CH
NH NH
CHOH -CHOH
That is a good precursor of the HE:
CHONO2-CHONO2
O2N-NH N-NO2
CH -CH
O2N-NH N-NO2
CHONO2-CHONO2
Also interesting alternative to formamide are carbamate ester CH3-O2C-NH2, (CH3)2N-CO-NH2, HO-NH2, NH2-NH2, NH2-CO-NH-NH2, NC-NH2, O2N-NH-CO-NH2,
O2N-N=C(NH2)2, (NH2)2C=NH and (NH2)2C=O!
     
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
O2NOCH-CHONO2
O2N-N N-NO2
CH -CH
O2N-N N-NO2
O2NOCH-CHONO2
This way it is better and correct!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hex
Harmless

Posts: 3
Registered: 19-6-2003
Location: Republic of Scotland
Member Is Offline
Mood: No Mood
|
|
An couple of alternative preps, not using TFAA are in GB Pat 1042905 (1966)
TFAA is often used in situations where having a non-nucleophillic anion as a by-product (ie CF3COO-) is an advantage. Don't forget that nitration
with TFAA or acetic anhydride works through CF3COONO2 or CH3COONO2, not nitronium ions - these species are not very good at nitrating N-acyl moeities.
Hey, Philou - long time! - don't think many of your alternatives to formamide would work, my friend...all those hydroxyls are too prone to
oxidation to have anything except an electronegative partial pi-bonded substituent on the nitrogen atoms. The urethane (H2NCOOEt) and the
N,N-diemthylurea might work, but I don't see much advantage.

|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
BTW, I made a rather serious nomenclature error; it should read
1,4-Diformyl-2,3,5,6-Tetranitratopiperazine
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
HHTDD !
ethylene glycol (being a dihydric) condenses with urea forming cyclic >CH<sub>2</sub>NHCONHCH<sub>2</sub>>
1,4-diformyl-2,3,5,6-tetrahydroxypiperazine (has 2 dihydric sides) may condense with urea. the resultant tricyclic needs to be deeped in conc
HNO<sub>3</sub>-H<sub>2</sub>O<sub>2</sub> then isolated and added to
HNO<sub>3</sub>-N<sub>2</sub>O<sub>5</sub> HOPING to get HHTDD (VOD~9700 m/s !)
edit: corrected the name for 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine.
[Edited on 7-9-2003 by KABOOOM(pyrojustforfun)]
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Ethylene glycol + urea is not a very easy way to get ethyleneurea. It works, but you need an autoclave or similar apparatus and it forms loads of
polymerisation products as well, apparently.
Unless you know of a different method..? Ethyleneurea is something I would love to get my hands on (EDNA...).
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
the PDFs on lagen's site have enough info on EU. I mentioned ethylene urea as an instant, not to be discussed here, DNethylene urea & EDNA
are interesting enough to make a thread about them. but here I wanted to discuss a new method of making
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatri-cyclo[7.3.0.0.]dodecane-5,11-dione(HHTDD).
|
|
|
Podrivnik
Harmless

Posts: 2
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Where this site?
| Quote: | Originally posted by KABOOOM(pyrojustforfun)
the PDFs on lagen's site have enough info on EU. I mentioned ethylene urea as an instant, not to be discussed here, DNethylene urea & EDNA
are interesting enough to make a thread about them. but here I wanted to discuss a new method of making
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatri-cyclo[7.3.0.0.]dodecane-5,11-dione(HHTDD). |
Where this site?
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
It was located here: http://lagen.kgb.cz/ However, the site has been down for several months at least. I knew I should of saved all those pdf files to disk.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
I have most of the files
btw is lagen still active on E & W?
|
|
|
Podrivnik
Harmless

Posts: 2
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
HHTDD
I am interested the information about synthesis of HHTDD.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Let's keep this on topic shall we?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
a little confused, as in another discussion it is stated that nitration of 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine leads to formation of the caged
explosive TEX.
http://www.sciencemadness.org/scipics/axt/tex-scheme.jpg
suppose it depends on the exact nitration conditions?
an alternate name could be:
1,4-Diformyl-2,3,5,6-Tetranitroxypiperazine
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|