lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Picric to Styphnic
I don't really mean for this thread to be "energetic" related, but I guess it is anyway.
I haven't been able to find any information really on the forum and I was wondering something.
Since picric acid is just a benzene with an NO2 at the 2, 4, and 6 position with an OH at the 1, and styphnic acid is the same, except with another OH
at the 3 (and im sure it could be at the 5 the same way).
So can a hydroxyl group be added to picric acid to yield styphnic acid?
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
If one could find OH+ it would be easy 
|
|
|
mantis
Harmless

Posts: 38
Registered: 17-7-2005
Member Is Offline
Mood: No Mood
|
|
you can try to brominate the ring-system of picric acid in a radical substitution. Now you can change it with a OH-Group in a nucleuphile substitution
with OH-.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Wouldn't that, maybe, neutralize the acidic picric acid in the process?
[Edited on 2/27/2006 by lacrima97]
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
Of course but you can reacidifiate the styphnate to stiphnic acid with conc acid and then wash.
BUT, I think bromination will yield more than styphnic acid... I'm thinking about trinitrophloroglucinol. Could be interessting though...
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
For some reason I am thinking that Bromine will not affect the 3, 5 positions because of some number....I can't remember exactly, but the numbers for
the 2, 4, 6 are 6-something, and the 3, 5 is 7-something. And for some reason I am thinking that once the phenol ring is configured the way that it
is in trinitrophenol, that it won't allow for any more additions for the ring. I'm sure what I have stated is incorrect, but for some reason I am
thinking this, yet I have no basis.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Indeed very hard to get something more on the ring...because of the negative effet of the nitro groups on the ortho and para positions and of the non
activating effect of the OH on the meta positions...
Speed of reaction will dus be very very slow.
It is much easier to nitrate a halobenzen than to halogenate a nitrobenzen...except with F2...
There is stil a possibility but it is tricky...because it would involve partial reduction of one of the nitrogroups into a aminogroups, then ring is
less desactivated. Then with a strong X(+) generator (example Br-F --> Br(+) + F(-)) and the right amount, you would add a halogen atom to the meta
position of the aminophénol (it being ortho or para).
Then with a peracid you must be able to oxydise the amino to nitro...Alternatively you could get it back by a diazotation reaction with CuNO2/Cu
powder.
The final step is the coolest...allow the compound to react with NaOH dilluted in water....this will lead to TNR
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Ohh, ok, I will study up on how to preform some of these reactions.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
I don't really know of anywhere else to put this, and I don't want to make a new thread, so....
Has anyone ever recrystalized picric acid from HCl to get the white form? I read in a book that the recrystalization of tnp from HCl will yield a
white form, and Im wondering if anyone has done it.
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
I have recrystalized TNP from K2CO3/HCl and dried with acetone... The results are bizare but at least, not white.
Check the thread http://www.sciencemadness.org/talk/viewthread.php?tid=870 (last post) for more info about my recrystalisation.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Why did you use K2CO3?
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
To get a soluble salt of picric acid.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
I am not asking of a salt, I was just wondering if anyone has ever made white picric acid from recrystalization from HCl.
|
|
|
ADP
Hazard to Others
  
Posts: 120
Registered: 4-4-2005
Location: USA
Member Is Offline
Mood: Productive
|
|
My crystals that I recrystalized from HCl differ from normally filtered ones. The crystals that are recrystalized from HCl are finer and have a more
pale yellow color to them as opposed to the lemon yellow of normal Picric.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by lacrima97
I am not asking of a salt, I was just wondering if anyone has ever made white picric acid from recrystalization from HCl. |
Sylla meant that he has first dissolved his picric acid in water as a salt...
2HO-C6H2(NO2)3 + K2CO3 --> H2O + CO2 (g) + 2 KO-C6H2(NO2)3
Finally by addition of strong HCl in water :
The weakest acid precipîtates...
KO-C6H2(NO2)3(aq) + HCl(aq) --> KCl (aq) + HO-C6H2(NO2)3 (s)
The reason of yellower picric acid by other cristallisation processes is obvious...basic or neutral media favourise the formation of the nitronic form
of picric acid what is deep dark orange...this was extensively explained elsewhere.
Thus partial proton jump from H-O to the vicinal NO2.
The raction is almost repressed in strong acid media!
The yellower the form the more sensitive it is.
White < Yellow < Orange
A orange form might be obtained from slow acidification/neutralisation of NH4 picrate with a weak acid.
 
[Edited on 7-3-2006 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by PHILOU Zrealone
The yellower the form the more sensitive it is.
White < Yellow < Orange
A orange form might be obtained from slow acidification/neutralisation of NH4 picrate with a weak acid.
 
|
About as likely as obtaining free phosphoric acid from
ammonium phosphate by treatment with vinegar .
Picric acid is a pretty strong acid and I just don't believe it
will be displaced that easily from ammonium picrate , or
from any other picrate , using any weak acid . It should
take a strong acid to do do the trick .
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
Could work if you find M and M' such that M-picrate is soluble but MM' isn't and HM' is a weak acid...
Wouldn't work with any alkali metal, they are all soluble. What about calcium picrate and oxalic acid ? I don't have any solubility tables around here
but if calcium picrate is soluble that should work (if your picric acid is free of sulfates)...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Or about as likely as to obtain HClO4 from the less acidic H2SO4 and sodium perchlorate...or HCl from H2SO4 and NaCl...
  
Don't forget that acidity tables are relatives...
HNO3 in water is acidic but in H2SO4 it is basic...because it produces OH(-) and NO2(+)!
To me everything under pKa= O in water is strong acid and really strong acids are under -2.
Picric is 0.5 a little more acidic than trichloroacetic acid and less than TFA acid.
OK it is commonly accepted that acetic acid (ethanoic) is an example of weak acid (pKa in water = 4.75)...So what is following you the range of pKa
for a weak acid?
Also the concentration is important...what is the most acidic?
95% HNO3 or 1% H2SO4?
Dont forget thus that acidity tables are an image of a statistic molecular dynamic process.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
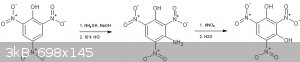
It might be possible afterall, by diazotization of 3-amino picric acid and reaction with water at higher temperatures to produce styphnic
(Attachment).
Did some experiments with the amination of picric to 3-aminopicric (3-ANP) using hydroxylamine as in US8404897 (attachement). Still optimizing the
process, but an interesting observation was that the use of relatively expensive lithium hydroxyde in patent US8404897 can seemingly be circumvented
by using zinc picrate/and NaOH (at least regarding solubility, whether the reported precipitation like as the lithium salt of 3-ANP is a necessity as
well remains to be seen). The zinc hydroxide formed initially dissolves in the very strong NaOH solution, so does not seem to interfere. 
Experimental:
In a 20 ml beaker, 1 g Picric was suspended in 10 ml dH2O. Then zinc carbonate was added at near boiling until a clear orange solution of zinc picrate
was obtained. This was cooled to room temperature and 0.8 g of hydroxylamine sulfate was added and stirred to dissolve. This was labeled solution A
and put aside.
To another 20 ml beaker, 3.9 grams of NaOH were weighed out and dH2O was added to a total volume of 10 ml. This was put in a cold waterbath (10 deg C)
and put on the stirrer plate. While vigorously stirring, the zinc picrate/hydroxylamine sulfate solution (solution A) was added slowly. No precipitate
of sodium picrate (as in most other reaction schemes using NaOH) or zinc hydroxide was formed, and a clear dark red solution was formed after only a
few minutes. The beaker was then covered with cling wrap to prevent air exposure. After 45 minutes at 10-15 deg C, still no precipitate had formed
(and time was running out the day), so HCl was added until strongly acidic, precipitating a decent amount of a greenish-yellow compound as fine
needles with melting point 160 C after recrystallization. Diazotizing this in 30% sulfuric produced a very acidic, light yellow compound of melting
point 170-180, deflagerating much like styphnic.
Curious why no precipitate of sodium 3-ANP was formed during the amination, is it really this soluble? What should be the colour of 3-ANP, the patent
mentions bright yellow? When using shorter reaction times, a dark orange-brown product is obtained, also with a melting point of 170-180, could this
be some interemediate meisenheimer complex instead? What are potential side reactions, introduction of 2 amine groups, reduction of picric, hydrolysis
of the nitrogroups by the strong NaOH solution? Hydrolysis of the aminogroup of 3-ANP, air oxidation?
IMO, the use of zinc picrate and NaOH instead of lithium salts as in US8404897 might prove to be more economical. Finding a very insoluble salt of
3-ANP might seem a good start, this could be added after formation of the very soluble meisenheimer complex and precipitate the 3-ANP directly from
the reaction mixture.
Attachment: US8404897 - Facile synthesis of 3-aminopicric acid - Copy.pdf (556kB)
This file has been downloaded 425 times
[Edited on 6-3-2018 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Tried the amination of picric on a larger scale this time:
All glassware and stirrerbars were thoroughly cleaned to make sure no residual copper or iron contamination from previous experiments was present.
Experimental:
Amination of picric acid to 3-amino picric acid:
2.3 g of picric acid were added to a 50 ml beaker, dH2O was added to 20 ml. The suspension was heated to near boiling and zinc carbonate was added in
small amounts until the pH was about 7, forming a deep orange solution. Water was added to 25 ml in total and the solution was cooled to around 20 deg
C (Cooling below 10 C will precipitate zinc picrate). Then 1.8 g of hydroxylamine sulfate was added and dissolved and the resulting solution put
aside.
To another 100 ml beaker was added 9 g of NaOH and water to a total volume of 25 ml. Once disolved, this was put in an icebath and allowed to cool to
0 deg C. While stirring strongly, the zinc picrate/hydroxylamine sulfate solution was added slowly using a pipette, while keeping temperature below 5
C. No precipitate was formed during the additions, a clear red solution formed immediately, changing to a darker red after only a few minutes. The
beaker was covered airtight in cling wrap and allowed to stand in the icebath for 5 hours in total. No precipitate was noticed, so tried adding 0.5 g
of KNO3, which also dissolved completely. After another hour in the icebath, a brownish precipitate was noticed, though too little to filter off.
Instead, about 25 ml of 50% cold sulfuric was added. During the final additions, the solution started to precipitate an abundant amount of
greenish-yellow needles so that the entire solution became momentarily nearly solid in appearance. A very small amount of NOx was noticed, presumably
from nitro group hydrolysis. The precipitate was filtered off, washed with cold water and immediately recrystallized from about 210 ml boiling 30%
ethanol. Upon cooling, 1.28 grams of a bright green-yellowish crystaline compound was isolated as feathery crystals, m.p. 170-180 C without
decomposition.
Diazotization:
*This is potentially a very dangerous procedure, never to be scaled up!*
100 mg of the bright green compound was added to a 20 ml beaker (in an improvised containement cabinet), about 1.5 ml of 70% nitric was added and
while stirring slowly, the suspension was gradually heated to 40 C. Only small amounts of NOx were released and very slowly, all of the 3-amino picric
went from green to an orange-red compound and finally went into solution, forming a clear dark red solution (likely of the diazonium salt). Water was
added to 15 ml and the solution heated slowly to about 80-90 C. Large amounts of an odourless gass were produced (likely mostly N2) and the solution
changed gradually from dark red to light yellow over the course of about 15 minutes. A light yellow precipitate was formed of melting point 170-180 C,
the potassium salt behaved identical to potassium styphnate.
So nothing terribly interesting it seems. Under acid conditions at least, the diazonium salt seems to behave as expected and nucleophillic
displacement of the diazogroup by water takes place to form styphnic. Nice excerise, but nothing fancy...
Still puzzled why no precipitate is formed during the amination and why the lithium salt from the patent posted above apparently does.
[Edited on 10-3-2018 by nitro-genes]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Has anyone on this thread considered nitrating resorcinol, I wonder ─ tfl;dr... 
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Direct nitration of resorcinol is troublesome...better go via the sulfonic acid or dinitroso derivative. 
Seriously though, using VNS for the amination of picric was just an cool reaction to perform. It seems also interesting that the substantial amount of
LiOH in the patent can possibly be replaced by zinc picrate/NaOH or lithium picrate/NaOH. The diazonium salt of 3-amino-picric is much more stable
than I thought and also interesting as a precursor to KDNP for example.
[Edited on 10-3-2018 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
It seems the internal diazo derivative of 3-amino picric (or the diazonium nitrate) can be precipitated after diazotization.
Experimental:
100 mg of 3-aminopicric was added to 1 ml 70% nitric at 0 deg C. Stirring slowly, solid sodium nitrite was added in excess, in very small increments.
The suspension obtained a dark red colour. After 20 minutes stirring, ice water was added to 15 ml total, precipitating a beautiful dark red (with
hint of purple) crystaline compound, that setted very quickly as a layer at the bottom of the beaker. A few ug's were isolated, it is very energetic,
single crystals puff off violently, almost detonating. It is pretty stable though, and does not seem to release much nitrogen when kept at 0 deg C,
even for an hour. Although hazardous at large scale, it seems possible to filter off and wash the diazocompound in extremely small quantities and
directy react it with NaN3 in 96-100% ethanol.
[Edited on 12-3-2018 by nitro-genes]
|
|
|