| Pages:
1
..
9
10
11
12
13
..
20 |
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
How many grams of acetylene in a #3 cylinder?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
A website says a #3 cylinder contains 75 ft^3 of acetylene. Without attempting to make any correction for temperature this would be:
wt. = (75ft^3)/(359ft^3/lb-mole)[(26 lb/lb-mole)][454g/lb] = 2466g
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
I assumed those numbers were the actual volume of the cylinder, I don't know how, it's obviously not...well if the cylinders are all filled by weight
why aren't those weights listed on the internets (they'll say the acetone weight).
I guess it's not bad if you already have a tank...or don't but want to make a lot of paraldehyde. I've always wondered why it's addictive.
[Edited on 10-10-2012 by S.C. Wack]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Acetaldehyde from Acetone
I looked in the search engine at the topics of acetadehyde- and I was surprised that no one brought up vapor phase oxidation of acetone. It sounds
like it is one of the most straight forward procedures, although I doubt that the yield would be good. However, with how cheap acetone is- why not
try!?
Check this out:
http://lecturedemos.chem.umass.edu/chemReactions5_4.html
The only thing that I can't quite figure out is how to capture the acetaldehyde that is formed. Perhaps we can condense it along with acetone, convert
it to the trimer, and reproduce te acetaldehyde?
Please reply if you have any ideas. Thanks!
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Nicodem
|
Threads Merged
21-11-2012 at 08:24 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Bisulfite adduct.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Substitution of Sodium Dichromate
As you all know, this week I am especially obsessed with acetaldehyde- and I would like to make some of my own 
This looks like the best and most productive way I've seen so far.
http://www.scribd.com/doc/45868796/preparation-of-Acetaldehy...
Problem- I don't have any sodium dicromate
Possibilities: I have ammonium dichromate. If I adjust the stoiciometry- could this work? I also have potassium chromate. I know acids convert this
into a dichromate. If K dichormate will work, can I just throw some extra acid in solution 1 and hope it works?
Thanks in advance!
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Nicodem
|
Threads Merged
22-11-2012 at 09:25 |
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
that looks like a half-assed school report. they list eight (8) synonyms for water..
[Edited on 22-11-2012 by tetrahedron]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
So? Even if it is a school report, it is a valueable resource. The author of this has quite a few other respectable documents. Of course, not as
venerable as an O-Chem text book, but in my mind- this procedure it describes is useful.
I have seen other sources that use dichromates to make acetaldehyde from acetone- so why do we have to declare that this is half-assed becuase it
lists 8 synonyms for water? 
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
It is half assed for other reasons. Personally I find the bizarre bulking up with tabular information about all the different compounds to be the most
objectionable; seven pages of mostly irrelevant info about water, H2SO4 etc. (including photos!) and then various sorts of critical information is
missing from the experimental. No sizes are given for any of the apparatus, no temperatures proposed or measured, no yield mentioned, no
characterization, no workup or purification mentioned, and of course whereas we can find a photo of a bottle of water for our report no photos of this
procedure are available. It's my assumption that the writer did not actually perform the experiment.
The less you bet, the more you lose when you win.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
That or he is a pretty sucky chemist! 
But us chemists like to experiment- We know that you can make acetaldehyde from this procedure, as stated in the very first post in this entire topic.
So, if we give this persons procedure the benefit of the doubt, perhaps we can make it better, and perhaps rewrite a scholarly paper on it!
[Edited on 24-11-2012 by ScienceHideout]
[Edited on 24-11-2012 by ScienceHideout]
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Acetaldehyde is unstable as anything. The best way to store it is as paraldehyde. Paraldehyde is also an effective sedative once used as a "Micky
Finn"and for historical (hysterical) reasons it's controlled. I would like a reasonable procedure for converting acetaldhyde to its polymeric form.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
ksj_6808
Harmless

Posts: 20
Registered: 6-7-2012
Member Is Offline
Mood: No Mood
|
|
Acetaldehyde is extremely volatile and cannot be stored satisfactorily unless it is refrigerated or sealed in glass ampoules (not just capped),
therefore it is necessary to prepare acetaldehyde each time it is needed. There is, however, an easier solution. A quick and easy way to store
acetaldehyde is to polymerize it to paraldehyde, which can be handled and stored easily, then depolymerize when you need to use it. Now you can make a
larger amount without worry.
To polymerize acetaldehyde for storage, place it in a dry test tube and cautiously add 1 drop of concentrated sulfuric acid per 2 mL of acetaldehyde
in the tube. Mix thoroughly, the polymerization will begin to take place. Some gentle warming can hasten the reaction. After some minutes add 3-4 mL
of water per 2 mL of acetaldehyde, an insoluble precipitate of paraldehyde will form. As an aside, technically paraldehyde is a controlled substance.
It is a sedative and a hypnotic drug useable by prescription only.
To depolymerize paraldehyde back into acetaldehyde, place the paraldehyde into a round-bottom 200-mL Florence flask. Add 4-5 drops of concentrated
sulfuric acid for every 20 g (20 mL) of paraldehyde in the flask. Set the flask up for fractional distillation, use glass in the fractionating column.
Use a 125-mL Erlenmeyer flask as the receiver; keep it cool by immersing in an ice water (but not salt-ice) bath. Place a loose plug of cotton into
the Erlenmeyer flask to help reduce evaporation loss; it must be loose. Care must be taken to prevent the cotton from coming into contact with the
distillate. After setting up, heat the flask gently. The temperature of the distillate must not be allowed to rise above 35 °C as it will only
repolymerize. The acetaldehyde is now ready for use.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Do you thing that we could oxidize ethanol with a hypochlorite or a peroxide? I was just thinking that calcium hypochlorite is right up at the top of
the oxidizer list near hydrogen peroxide and chromates. If we can oxidize it with a chromate, is it possible that one of the mentioned compounds would
work? We can then distill off the acetaldehyde, and polymerize it.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Nah, those are much too aggressive. Chromate is milder than either; PCC I believe is preferred further still.
Tim
|
|
|
killer_lapin
Harmless

Posts: 47
Registered: 23-7-2010
Location: Qc, CAN
Member Is Offline
Mood: No Mood
|
|
Earlier they mention this technic but they were proposing to oxidise ethanol instead. The difference seems that the acetone is easier to oxidize than
ethanol, perhaps the copper catalyst doesn't need to be activated before use.
Quote: Originally posted by Organikum  |
Dehydrogenation of EtOH:
Coppercatalysts with ZnO, CoO and CrO3 as structural promotors. The reaction temperature is limited to 270°C - 330°C for to reach a selectivity
towards acetaldehyde of 95%.
Yields (per pass) are limited to 30% to 50% this way.
The hydrogen produced as byproduct is clean enough for use in catalytic hydrogenations.
The preparation of the catalyst is a standard procedure for precipitating metal catalysts onto a metal support - I think I got it from some patents.
It is tried and true.
(it cost me a damned long time to find out what "structural promotors" means)
If you precipitate copper on zinc you will see that the copper grows on the zinc in form of fractal "trees" providing a huge surface area later in the
dehydrogenation. Thats the whole trick of the "structural promotion" of zinc.
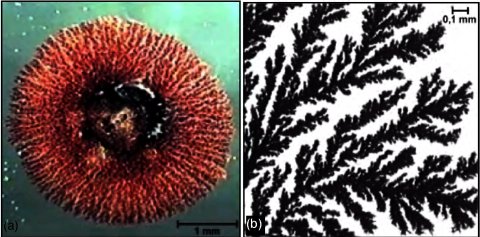
A coppertube filled with copper-scrubpads works fine if the amounts of acetaldhyde needed are not to large (less than one liter) - important is to
activate the copper by first oxidising it - blowing air through the tube at red dull heat - and reducing it again what can be conveniantly done by
passing ammonia or hydrogen through the hot tube.
It actually works also without this activation step. but yields are low. |
For a setup chek here:http://www.sciencemadness.org/scipics/tubefurnace_etoh.jpg
|
|
|
blue_vitriol
Harmless

Posts: 11
Registered: 28-12-2012
Member Is Offline
Mood: No Mood
|
|
Has anyone tried using MnO2? If that would work it would be extremely convenient, because it can be extracted from batteries.
|
|
|
killer_lapin
Harmless

Posts: 47
Registered: 23-7-2010
Location: Qc, CAN
Member Is Offline
Mood: No Mood
|
|
MnO2 is not powerfull enought. Permanganate could do the job but it's probably to powerfull. But electrogenerated permanganate could work.
|
|
|
Nitro-esteban
Harmless

Posts: 39
Registered: 10-4-2013
Location: Fifth dimension
Member Is Offline
Mood: inert
|
|
Quote: Originally posted by Polverone  | Navarone, Navarone's Ghost, Anubis:
Your writing is sloppy, riddled with errors, and provides little of interest even when one ignores its technical blemishes.
I have valuable advice for you:
The next time you post anywhere other than Whimsy, provide enough details to show that you've already investigated the topic you are talking about.
The investigation could be an experiment that you have conducted, or that you have seen someone else conducted, or even something that you read in a
book or journal or on a reputable website.
Whatever you post about, you should make a respectable effort to spell words correctly and in their entirety, and to use such perfect punctuation and
grammar that no 7th grade teacher of English would hesitate to give you an "A."
If you don't post about a technical subject, and don't post in Whimsy, then your prose should be rich and beautiful in addition to technically
flawless. If Vladimir Nabokov's ghost isn't green with envy, you have failed.
I hate cluttering up perfectly good threads with this sort of off-topic discussion, so let me be extra-clear:
If you are incapable of following the above advice, kindly go home and eat bleach and die. |
I think bleach is composed mainly of hypochlorites which are probably not toxic enough to kill someone.
|
|
|
Salmo
Harmless

Posts: 42
Registered: 20-9-2012
Member Is Offline
Mood: No Mood
|
|
what do you think about starting from choline, should be OTC, and than proceed with a hofmann elimination?
http://en.wikipedia.org/wiki/Choline
http://www.amazon.com/NATURES-WAY-Choline-500mg-CAPS/dp/B000...
http://en.wikipedia.org/wiki/Hofmann_elimination
[Edited on 5-6-2013 by Salmo]
|
|
|
Nitro-esteban
Harmless

Posts: 39
Registered: 10-4-2013
Location: Fifth dimension
Member Is Offline
Mood: inert
|
|
Could potassium permanganate be used to oxidize ethanol or other alcohols? I have plenty of it but it is a carcinogen so I don't want experiment with
it unless there is a good chance that it will work.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nitro-esteban  | | Could potassium permanganate be used to oxidize ethanol or other alcohols? I have plenty of it but it is a carcinogen so I don't want experiment with
it unless there is a good chance that it will work. |
Permangenates mixed with alcohols often spontaneously combust, so while you can oxidize alcohols with permangenate, you often will over-oxidize them
to acetic acid or CO2, rather than stopping at acetaldehyde, unless you carefully control the conditions. These reactions are normally done dilute,
in an inert (to oxidation) solvent to keep the rate slowed down, otherwise they can heat up, accelerating the reaction in an uncontrollable manner.
Even with PCC it can be hard to keep low MW aldehydes from overoxidizing unless you use carefully tuned conditions. But that will usually work if
they are dilute, the reaction is not allowed to overheat, and the PCC is added slowly.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
There are several highly selective reagents that can oxidize alcohols to aldehydes, and not any further.
But for just making acetaldehyde, these reagents are probably not practical.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Should acetaldehyde be possible to produce via similar method as formaldehyde: catalytic oxidation of ethanol or acetone with oxygen on copper, silver
or palladium catalyst at high temp?
Im gonna run some tests on aldehydation process by making copper pipe spiral installed in heat shield on a propane heat source and distill some
methanol, ethanol and acetone through a copper pipe and pumping air with it to induce catalytic reaction.
Products with ethanol and acetone should contain acetaldehyde and ethanoic acic, selectivity varying by the degree of oxidation where faster speeds
through catalytic reformer and lower oxygen amounts prefer formation of acetaldehyde and higher oxygen amounts and longer catalyst times prefer the
formation of secondary oxidant, ethanoic acid. With methanol the corresponding products should be formaldehyde and formic acid with similar
conditions.
The recovery of the products could be done with two receiver flasks, where with acetaldehyde/ethanoic acid the first one serves a receiver for acetic
acid, heated at 20C and secondary for acetaldehyde, cooled with ice-salt bath, and with formaldehyde, the primary container holding the formic acid,
and secondary containing a water with pipe underneath it to form a formaline solution up to 40%.
[Edited on 4-7-2013 by testimento]
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
You did not bother to read the thread as it seems but you seemed to actually do something and so one click might be not to much to enlighten you about temperatures and catalysts for such a process.
regards
/ORG
edit: wow! I just saw you did not even read the last page, now thats a bit daring my dearest...
and all these posts, will be lost, like tears in the
rain...
[Edited on 4-7-2013 by Organikum]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
"Acetaldehyde is extremely volatile and cannot be stored satisfactorily unless it is refrigerated or sealed in glass ampoules (not just capped),
therefore it is necessary to prepare acetaldehyde each time it is needed. There is, however, an easier solution. A quick and easy way to store
acetaldehyde is to polymerize it to paraldehyde, which can be handled and stored easily, then depolymerize when you need to use it. Now you can make a
larger amount without worry."
Nope, I personally used to own a big glass bottle of it. I don't remember any special problems with it. It is a low boiler, but at normal
temperatures, it did not seem to escaped containment, nor did it develop enough vapor pressure to burst the bottle. It would probably be nice to keep
it cool in a refrigerator, but it certainly wasn't shipped to me under refrigeration.
|
|
|
| Pages:
1
..
9
10
11
12
13
..
20 |