| Pages:
1
2 |
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Metallic hydrogen
There's an interesting article on Wikipedia about metallic hydrogen:
http://en.wikipedia.org/wiki/Metallic_hydrogen
Basically, if you squeeze hydrogen to insane pressures, you can theoretically convert it to a metal with a relatively high density. There's
some evidence that it has been prepared in very small amounts, though the field is still very juvenile.
The tantalising thing about metallic hydrogen is that there is a fair chance that it would be metastable at low temperature, much like diamond for
example would only convert to graphite upon strong heating because of a kinetic barrier. Now certainly the metallic hydrogen bond will not be nearly
as strong as the carbon bond, hence this conversion temperature may occur at much lower temperature, but nevertheless.
The thing is, diamond, in spite of being extremely thermodynamically unfavorable to form at low pressure, can be made with vapour deposition methods
in a vacuum... even large gem quality stones (see wiki article on "synthetic diamond")!
The question beckons then, maybe metallic hydrogen could be made at low pressure, using low temperature to prevent it from 'reverting' to diatomic
hydrogen?
Metallic hydrogen, if it indeed would be metastable, could be the ultimate chemical fuel and fully enable the hydrogen economy.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Wow! Intriguing.
There are a lot of unknowns though. Chiefly metastability is only speculated. And even if this was the case, synthesis in the quantities necessary for
a fuel is likely to be a significant hurdle.
It's a bit like fusion IMO.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Some applications, like the theoretical room temperature superconductivity mentioned in the wiki entry, would
warrant production even if expensive 
Also, even if it's not metastable at STP... it could be made inside single-walled carbon nanotubes (SW-CNTs) to contain it, perhaps. I recall reading
about using SW-CNTs as nano gas cylinders to store gaseous hydrogen at very high pressure 'capped' with ice at their ends and then simply defrosted to
release the H2. [1] Granted, this would be much higher pressure, but it could help 'slow' the kinetics of hydrogen conversion from metal to gas,
perhaps slow it a lot.
SW-CNTs are incredibly strong and become diamond-hard at gigapascal pressures AFAIK.
I've already posted elsewhere [2] recently about the discovery that graphene freely permits protons from diffusing through it while not H2, while the
claims that this could permit the harvesting of hydrogen from air is pseudoscience in my opinion, the science of the proton diffusion is not.
From this discovery, I presume that protons probably have a good chance of diffusing through single-walled carbon nanotubes as SW-CNT is just graphene
rolled-up. Now one just needs to find a way to reduce the H+ inside a nanotube and you'll be in business 
?electrochemical? Though one would need to stop the outside of the CNT's from being conductive, only the inside
should conduct so that the H+ reduces on the inside.
[1] http://www.materialsviews.com/tiny-tubes-hydrogen-storage-in...
[2] SM topic "Harvesting hydrogen from air... free energy with graphene?"
[Edited on 29-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by deltaH  |
...
The tantalising thing about metallic hydrogen is that there is a fair chance that it would be metastable at low temperature, |
Maybe. The history of metallic hydrogen experiments though shows that efforts to calculate its properties from quantum principles has not been very
reliable - transition pressures seem much higher than originally predicted, and the first indications of metallic transition occurred in high
temperature conditions when it was not expected (the LLNL shock experiments in the 1990s). Perhaps it would be best put that "the possibility cannot
be excluded".
| Quote: |
The thing is, diamond, in spite of being extremely thermodynamically unfavorable to form at low pressure, can be made with vapour deposition methods
in a vacuum... even large gem quality stones (see wiki article on "synthetic diamond")! |
Extremely? Care to cite some comparisons to defend this choice of superlative?
The energy difference between graphite and diamond is about 2 kJ/mole. Lots of materials have heats of fusion larger than this.
Graphite is certainly favored, but I think you may be exaggerating how unusual this situation is.
| Quote: | | The question beckons then, maybe metallic hydrogen could be made at low pressure, using low temperature to prevent it from 'reverting' to diatomic
hydrogen? |
Do you have any literature to cite where this is thought to be a possibility? I have followed this subject on a casual basis for a few decades and
have never seen it suggested.
For metallic hydrogen the energy difference is 400 kJ/mole, not 2.
Analogies don't work very well in physics.
Updated Remark:
This is the difference, and why low pressure metallic hydrogen formation will never happen.
Diamond is in a higher energy state than graphite, and thermodynamically would release 2 kJ/mole if it transformed into it (but this never happens,
you have to melt the diamond lattice first). But relative to carbon vapor at the same temperature it is in a lower energy state. All CVD does in
deposit carbon on a patterning surface that prevents it from spontaneously forming its lowest energy hexagonal lattice. The carbon is still giving up
energy to form the lattice either way, it just gives up a little less to from diamond. Both diamond and graphite have negative energy stored relative
to the atomic or cluster form.
Metallic hydrogen is interesting because it has a huge amount of positive energy stored in its lattice, relative to any other form of hydrogen.
Conversion of metallic hydrogen into atomic hydrogen is supposed to release a huge amount of energy.
There is no way a low pressure process can store that energy in the bonds as the metallic hydrogen forms. Only high pressure that forces the atoms
closer together than they want to go can do that.
End Remark
Here is better summary of the state of the science on this:
http://www.nature.com/news/metallic-hydrogen-hard-pressed-1....
[Edited on 29-12-2014 by careysub]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
@careysub,
The fact that it formed at high temperatures makes intuitive sense to me by the analogy to carbon: diamond having been initially made synthetically at
high temperature and pressure. There's a nice cited graphic of the phase diagram of carbon with an overlay of the various synthetic diamonds made.
See:
http://www.ch.ic.ac.uk/rzepa/mim/century/html/diamond_text.h...
Carbon's phase diagram was my basis for saying it's "extremely" thermodynamically unfavourable because of the large magnitude of the P-T values
defining the classical diamond region... but in hindsight, a poor choice of words, particularly at low temperature!
To convert H2 to H(m), you need to break the H-H bond and this is a significant kinetic hurdle, so it's not a surprise that high temperatures are
helpful. The use of catalysts to aid this at lower temperatures could be beneficial and probably already being/have been tried for metallic hydrogen?
However, the 400kJ/mol figure the metallic hydrogen transition is very high and about the same as hydrogen's bond dissociation energy of 436kJ/mol[1].
Since one would still need to subtract the energy of condensing to the metallic state or in effect the metallic bond dissociation energy, I'm
cautiously sceptical of this 400kJ/mol figure. Then again, by your own arguments, modelled values of metallic hydrogen have not proven very reliable.
Also, there's no reason to suspect that the hydrogen metallic bond would be very weak, on the contrary, it's probably quite strong if one looks at the
trend going up the group in heats of vaporization of the alkali metals in the periodic table.
lithium: 136kJ/mol;
sodium: 97.42kJ/mol;
potassium: 76.9kJ/mol;
rubidium: 69kJ/mol;
caesium: 63.9kJ/mol;
This fits very nicely on a polynomial of degree 3  so projecting backward to
hydrogen, one would predict a dH vap of +-200kJ/mol H or 400kJ/mol for 2H. so subtracting this from the H-H bond dissociation energy, I would get an
estimated value of the heat for the metallic hydrogen transition to be a mere 36kJ/mol... granted, by extrapolations, but nevertheless, not 400kJ/mol so projecting backward to
hydrogen, one would predict a dH vap of +-200kJ/mol H or 400kJ/mol for 2H. so subtracting this from the H-H bond dissociation energy, I would get an
estimated value of the heat for the metallic hydrogen transition to be a mere 36kJ/mol... granted, by extrapolations, but nevertheless, not 400kJ/mol

As for your literature request, I do not have literature to support that it could be made at low pressure and temperature. Since I was basing it on an
analogy of another allotrope phase change, that of carbon, it was merely a suggestion that this might be a future development. Since the
thermodynamics of the hydrogen metallic state is unknown, I cannot make further predictions about it. That is not to say that one might not be
inclined to experiment with it in the hopes of achieving a breakthrough  However, this might be as foolish and as impossible as achieving alchemy, though we will only know once the system is better studied.
However, this might be as foolish and as impossible as achieving alchemy, though we will only know once the system is better studied.
The bottom-line is that I placed this in the "beginnings" section because it is just that. This is an emergent technology (in terms of it having been
prepared) of which there is not a great deal known just yet. However, I have a sneaky suspicion that there will be a steep developmental curve soon.
[1] http://en.wikipedia.org/wiki/Bond-dissociation_energy
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hypothetical cell for producing metallic hydrogen
Here is a schematic of my hypothetical apparatus for attempting to make metallic hydrogen electrolytically. Running on the hypothesis that
there is a significant kinetic energy barrier to converting H(m) to H2(g) at low temperature, meaning that metallic hydrogen could be metastable at
low temperatures, then I propose the following electrolytic cell for preparing it:
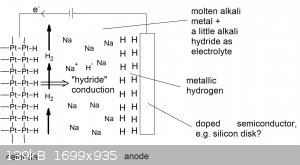
Let me explain the reasoning behind the design.
The way I see it is that metallic hydrogen would behave as a very electropositive metal, probably the most electropositive metal if one looks
at the reduction potential trend of the alkali group.
So if one were to electrolyse an alkali metal salt in a protic solvent, you
would form H2, not the pure alkali metal.
This is because the hydrogen half-reaction has a standard electrode potential of zero while the alkali metals are very positive.
Now a metallic hydrogen would be even more electropositive (most likely), that means that this reaction at a cathode of a conventional electrolysis
cell forms H2(g), what we all know. Put another way, hydrogen metal probably reacts as any electropositive metal would with an acid... producing H2.
Ok, chemical kinetics would determine rate which might be fast or slow, but that's beyond what I can speculate on at this stage.
So now, how to exclude protic solvents but still deliver H to an electrode in electrolysis?
I suggest using something similar to electroplating and with a hydride as the electrolyte.
At the cathode, hydrogen gas is bubbled over a platinum wire/gauze/foil. The hydrogen is dissociated catalytically on the surface of the platinum to
H* which is then ionised by addition of an electron to form H-.
This is then attracted to the anode of the cell where H- is oxidised to not hydrogen gas, but metallic hydrogen, on a non-catalytic electrode.
Now the problem comes in the selection of solvent and electrolyte. Molten LiH, for example, is very hot at 689°C. This is too hot for any chance of
preserving the metallic hydrogen state. Indeed, this has been done and it's been found to produce H2(g)... no surprise [1].
But what if you did this at close to room temperature. Unfortunately, alkali hydrides are not soluble in aprotic inert solvents, but they NaH is
soluble in molten sodium. [2]
So low melting liquid alkali's are potentially a 'solvent' for this system, except there is a HUGE problem... spotted it yet?
Molten metals conduct electricity very well indeed 
So in such a cell, there would be a short and you could not maintain the required potential difference to reduce the H2(g) to H- and oxidise the H- to
H(m) (if that's even possible).
My suggested workaround for that is using a semiconductor for the anode. That way you form a Schottky junction (metal to semiconductor) that allows
one to maintain a potential difference on the cell. Hopefully, the voltage drop of the Schottky junction is large enough to bring about the oxidation
of the hydride.
Potentially one could also use NaK liquid metal to run this cell at room temperature.
NB: I do not know if platinum or silicon is attacked by Na(l) or NaK for that matter and there are many many other ??? for this, but I'm just trying
to start to think about this topic in terms of potential ideas for making metallic hydrogen in unconventional routes.
That's about the best I could think of to give metallic hydrogen the best chance it could have to form, at or near STP, if it is even
thermodynamically possible in the first place?
[1] http://www.nrcresearchpress.com/doi/pdf/10.1139/v58-215
[2] http://en.wikipedia.org/wiki/Sodium_hydride
EDIT: Additional point to add
Just realised that the hydrogen could be 'soluble' to a degree in the molten alkali, so the molten alkali should be saturated with hydride or run
the partially saturated cell for some time to form additional sodium hydride.
[Edited on 29-12-2014 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  |
The way I see it is that metallic hydrogen would behave as a very electropositive metal, probably the most electropositive metal if one looks
at the reduction potential trend of the alkali group.
|
Nope. What the trend shows is that of the Group I elements (I'm old school and include hydrogen in that group), hydrogen is the least
electropositive of all of them.
Somewhat arbitrarily, hydrogen's reduction potential (of 0 V) in the reduction potentials series marks the demarcation between the electropositive
elements and the 'not so electropositive' ones.
Those in that first 'group', as metals, they can be oxidised by H<sup>+</sup> (well, H<sub>3</sub>O<sup>+</sup> , the latter group cannot. , the latter group cannot.
[Edited on 29-12-2014 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
@blogfast
Hydrogen gas has a reduction potential of zero, not hydrogen metal, that would have a different potential. Its reduction potential is unknown
for the time being. I am going by the trend that as you move up the group in alkali metals, the reduction potentials get larger and larger so that a
metallic hydrogen would be the largest.
EDIT: Aditional comment
I also think metallic hydrogen can be oxidised by H+ to H2(g), that's why I have to avoiding it in my cell and working with hydrides instead 
[Edited on 29-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Although there is a lot about the properties of metallic hydrogen [H(m)] that remain uncertain, many years of quantum calculations are making progress
in clarifying the possibilities and there has been recent very important work.
The overall picture of how hydrogen progresses to the metallic atomic phase is becoming fairly clear - it is hardly that case that nothing is known
about it.
The latest work in this area indicates that the zero temperature transition to this state occurs at 5 megabars:
http://arxiv.org/pdf/1011.5028.pdf
That metallic hydrogen contains a great deal of 'strain' energy, in addition to the latent hydrogen bond energy, is necessary from simple Newtonian
considerations. The work done in moving hydrogen along the zero temperature PV EOS curve (the integral of PdV) must be present in the final material.
A 12-fold reduction in volume while going up to 5 million atmospheres necessarily stores a large amount of energy. You would be correct in doubting
any precise figure offered, since the precise curve is not known, but any possibility yields values of >100 kJ/mole.*
How could this energy could be deposited incrementally on the surface of a growing specimen in a vacuum situation a la CVD? The situation is nothing
like the growth of a diamond crystal.**
While the strain energy is a dominant first order effect, the question of metastability is not. It requires a number of second order effects to sum up
properly for metastability to exist (and arguments about quantum tunneling at the surface not to be correct). It may exist, but even our best
calculational tools cannot settle the question today. (Cup half full version - neither can they rule it out.)
Regarding efforts to extrapolate thermodynamic properties directly from the alkali metals - lots of factors that are negligible with stable STP
materials are not negligible with metallic hydrogen, hence the calculational difficulties (metastability, if it exists, depends on the influence of
zero point energy!).
*This same argument applies to compressing carbon also, but the zero temperature transition pressure between stable graphite and stable diamond is
very low as high pressure research goes, only about 15 kilobars, and the density ratio is also much smaller, so the potential for storing energy in
this way is very much smaller.
Also, it impossible to get diamond by zero temperature compression of graphite, you only get compressed graphite which returns to its former state
when the pressure is removed, so the situation with regard to H(m) is not parallel. Shock experiments that produce diamond from graphite create
temperatures above 5000 K, thus melting the graphite lattice.
**The surface tunneling effect would seem to undermine any scheme to build the material up in layers, even if the problem of adding energy to overcome
repulsion could be addressed.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
@careysub
I don't understand you completely and I might be viewing this through the lens of a chemist, but honestly, I just don't see how hydrogen can gain so
much energy in metallization when formed at STP. They're still a type of hydrogen-to-hydrogen bond/s, just of a different type and spread out.
Allotropes in general do not have very large dG's AFAIK, if you know of any specifically, please suggest. I thought carbon was the worst case in terms
of dGf between allotropes, but I haven't looked it up?
I also don't see why my analysis of using the heats of vaporization trends and the bond dissociation energy of hydrogen is not a reasonable
back-of-the-envelope estimation of metallic hydrogen's dGf at STP... NB: not at extreme pressures where there would be additional lattice
strain and potential energy!
Also, are phase transitions not activated processes? If so, then the issue of metastability is implied IMHO. It must be metastable at some cold
temperature where the kinetics are just too slow to be of consequence... the question is whether this could be at room temperature.
Even if not metastable at room temperature, then there are other strategies to contain it as I've suggested, like 'bottling' it in carbon nanotubes...
provided one can form it within them, which is another matter altogether 
Finally, I agree and don't see how CVD could be possible for making metallic hydrogen neither.
[Edited on 29-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by deltaH  | @careysub
I don't understand you completely and I might be viewing this through the lens of a chemist, but honestly, I just don't see how hydrogen can gain so
much energy in metallization at STP. |
It can't. It does not happen at STP. It might happen at ST (273 K) but P is 5 million times higher than atmospheric.
Metallic hydrogen comes into existence when high pressure causes the normal outer electron shell structure to collapse, freeing the electron to roam -
basically pressure-induced ionization. All substances will become metallic at sufficiently high pressure, hydrogen in not unique in this way. It is
unique in that that "outer electron" is the <i>only</i> electron.
The issue of metastability comes up because once formed, it might be possible to depressurize it to STP or thereabouts (where it would be useful)
without it reverting to ordinary hydrogen. It is not known whether this can occur (but we also don't know that it can't).
Despite people using diamond as an analogic case of something similar happening in a familiar situation, it is in most ways misleading. We have no
examples of a material being transformed into a new state at megabar pressures, which survives depressurization to STP.
Hydrogen is not an alkali metal -
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Which is why you should not form it at those high pressures and pre-charge it with so much potential energy.
If I understand you correctly, you are suggesting that using high pressures is the only way to form it? In that case, I agree that low-pressure
methods probably won't work.
If I simply took the high pressure modelled latice of metallic hydrogen and performed a ab initio calculation at 0K, 0 added stress, are you
saying that optimising the geometry will necessarily form solid hydrogen as we know it (with short H-H bond lengths in the lattice and low density
overall)? Or would it simply relax a bit but still minimise to a metallic state.
Perhaps this has already been published and someone can shed light on the matter.
EDIT: I am still to work through your paper, too late to do that today, need sleep 
[Edited on 29-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by deltaH  | Which is why you should not form it at those high pressures and pre-charge it with so much potential energy.
If I understand you correctly, you are suggesting that using high pressures is the only way to form it? In that case, I agree that low-pressure
methods probably won't work.
[Edited on 29-12-2014 by deltaH] |
Yes, that is correct. Metallic hydrogen is predicted from high pressure conditions, originally by Wigner and Huntington at the (much too low) pressure
of 250 kilobars. It is early result of quantum mechanical theoretical chemistry.
http://adsabs.harvard.edu/abs/1935JChPh...3..764W
http://www.tandfonline.com/doi/abs/10.1080/08957959.2013.784...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I'm not so sure about that, the predictions you speak of are the pressures where the modelled energies of the metallic state are lower than the solid
H2 state, but I see no evidence or explanation to suggest that the structure cannot be modelled at 0Pa and 0K.
In the molecular modelling paper you linked above, you can see this on the plots in figure 1. If you look at the low-pressure region, you see all the
metallic H lines starting from some arbitrarily human chosen starting point. The author probably reasoned that there is no need to focus on the
lower region since the lines lie above the solid H2 line there and so is thermodynamically unfavoured... but those structures probably can be modelled
at 0 Pa and 0K.
Their I4/amd structure, the lowest energy one, is one found by search a wide range of structures, but it might not be THE most stable. It is itself
completely theoretical. We have no idea that there isn't a more stable low temperature/pressure metallic hydrogen structure. It's an educated guess.
While the structure is probably accurately predicted by the calculation, what is much less certain is if it's THE structure in the first place in the
STP region.
On top of that, there are other effects (such as the spin configuration of the hydrogen) that can change energies further, particularly at low
temperature.
The problem with ultra high pressure material science/physics is that its self-limiting. In a way you have alluded to this already, make them at
extreme pressures and you tend to focus on only considering that high-pressure domain.
Diamond had exactly that problem, for a very long time, people only focussed on the high-pressure region until a technology (CVD) suddenly
demonstrated that they could form even in vacuum.
Your argument is that the energy differences between specifically metallic hydrogen and solid hydrogen is too large to permit this, but this is a
educated speculation. A simple other educated speculation, my back-of-the-envelope estimation, suggests otherwise and intuition suggests that as with
other element's known allotropes, energy differences at STP are not that large... why should hydrogen be super different at STP?
Anyhow, I don't yet see anything that suggests it's impossible to form it under milder conditions... it's an open question IMHO.
That said... I'm still probably wrong 
[Edited on 30-12-2014 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
deltaH:
Here are the Pauling electronegativities and 1st Ionisation energies (kJ/mol) respectively, for the Alkali metals:
Li.....0.98.....520.2
Na.....0.93.....495.8
K.....0.92.....418.8
Rb.....0.82.....403
Cs.....0.79.....375.7
The trend is clear. And easy to explain: as N (the Period Number) goes up, the Ns<sup>1</sup> electron is further and further away from
the (shielded) nucleus and thus easier to remove.
By your own reasoning metallic H should have the highest electronegativity value (Pauling) and the highest first ionisation energy, at least in
comparable conditions. In 'normal' hydrogen the 1s<sup>1</sup> electron is so tightly bound to the nucleus that hydrogen forms covalent
bonds mostly, rather than ionic ones.
And I can't for the life of me conceive of metallic hydrogen except in these ultra high pressure/low temperature regions...
[Edited on 30-12-2014 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
My bad, you are correct blogfast, I was thinking of the standard reduction potentials with lithium being at the top in terms of
having the largest positive Ecell value of the alkali's, silly error, my apologies... too busy thinking of my crazy cell designs 
As trends go, generally the alkali's get less reactive going up, but I don't think hydrogen in a metallic state will hold to that because of the extra
'kick' of its positive dGf. Also the metallic hydrogen bond is probably far easier to break (weaker) than H-H bonds, so it would also probably appear
kinetically far more reactive as a reducing agent.
I think one could safely say that you could probably use it as a reducing agent as is if it were metastable, without the need for catalysts, though
that would be such a waste. Now there's a thought... reduction of nitrostyrene with metallic hydrogen   
[Edited on 30-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by deltaH  | | I'm not so sure about that, the predictions you speak of are the pressures where the modelled energies of the metallic state are lower than the solid
H2 state, but I see no evidence or explanation to suggest that the structure cannot be modelled at 0Pa and 0K. |
Of course they are lower than the solid H2 state - at or above the pressure of transition. Otherwise the phase transition to the metallic state would
not occur. But the internal energy (and the Gibbs free energy) of the high pressure H2 solid state are enormously higher than the STP solid H2 state.
That is the explanation of why it cannot occur at zero pressure.
| Quote: | In the molecular modelling paper you linked above, you can see this on the plots in figure 1. If you look at the low-pressure region, you see all the
metallic H lines starting from some arbitrarily human chosen starting point.
The author probably reasoned that there is no need to focus on the lower region since the lines lie above the solid H2 line there and so is
thermodynamically unfavoured... but those structures probably can be modelled at 0 Pa and 0K. |
This is difficult to respond to, since there is no actual argument or reference to any factual information in this paragraph.
I cited some of the latest work in the field to show the high level of sophistication the work has reached with modern computational tools and theory
- in answer to your implications that no one in the field really knows anything about this.
Clearly the effort failed since your response is still "my wild guesses are probably right while the experts are wrong" even though you cannot support
it with anything at all.
Are you familiar with the "Dunning–Kruger effect"?
This field of study has been actively pursued for 80 years by some of the most brilliant minds in physics (starting with Wigner, others include
Metropolis, Bethe, Feynman) and the general outline of the physical situation has been clear from the beginning - that an enormous amount of internal
energy is necessary to free the hydrogen electron from being bound to a proton so that the metallic state can exist.
| Quote: | | Their I4/amd structure, the lowest energy one, is one found by search a wide range of structures, but it might not be THE most stable. It is itself
completely theoretical. We have no idea that there isn't a more stable low temperature/pressure metallic hydrogen structure. It's an educated guess.
While the structure is probably accurately predicted by the calculation, what is much less certain is if it's THE structure in the first place in the
STP region. |
You are hoping here that refinements of third-order effects, which determine the precise lowest energy structure might make the huge first order
effect disappear.
Sorry, not a plausible argument.
| Quote: | | On top of that, there are other effects (such as the spin configuration of the hydrogen) that can change energies further, particularly at low
temperature. |
It is all these other third order effects that hopes of metastability at STP might exist are based on. But this can in no way can make the enormous
internal energy requirement that all structures share go away.
| Quote: | The problem with ultra high pressure material science/physics is that its self-limiting. In a way you have alluded to this already, make them at
extreme pressures and you tend to focus on only considering that high-pressure domain.
Diamond had exactly that problem, for a very long time, people only focussed on the high-pressure region until a technology (CVD) suddenly
demonstrated that they could form even in vacuum. |
Again the diamond analogy as an argument. The graphite/diamond situation is so profoundly different from metallic hydrogen that it is far more
misleading than helpful.
Consider: both diamond and graphite have the same covalent bonds, with only a small energy difference (2%). The thermodynamics of vacuum deposition of
diamond is scarcely any different from graphite. The zero temp stability boundary is only barely in the "high pressure" regime at all, a mere 15
kilobars. Graphite and diamond are (comparatively speaking) practically the same.
Metallic hydrogen requires the shell structure of the hydrogen atom to be completely disrupted and replaced with a different bonding mechanism - the
electron has to be freed from the nucleus. Pressure ionization on the order of 5 megabars, over 300 times higher than the (from this perspective)
low-pressure graphite-diamond boundary. There is no analogous example of this you can cite with any zero pressure allotrope change. Not diamond.
Nothing.
| Quote: | | Your argument is that the energy differences between specifically metallic hydrogen and solid hydrogen is too large to permit this, but this is a
educated speculation. A simple other educated speculation, my back-of-the-envelope estimation, suggests otherwise and intuition suggests that as with
other element's known allotropes, energy differences at STP are not that large... why should hydrogen be super different at STP?
|
Because metallic hydrogen is super different.
What is the largest condensed matter zero pressure allotrope molar volume change you know of?
I think it is the graphite/diamond ratio which is 1.6, which also has the lowest molar volume of an allotrope of any element at zero pressure - a
value of 3.4. (They range up to a high of 74.1 for Cs).
To form metallic hydrogen normal condensed hydrogen has to have it its density increased 12-fold, far above the density change of any zero pressure
allotrope. It ends up with a molar volume of only 0.9, about four times denser than any low pressure atom packing.
I don't know what BOTE calculations you did, but I can't agree that they qualify as an "educated speculation", and implying that it is equivalent in
stature to the results of 80 years of quantum chemistry research is just... strange.
| Quote: | | Anyhow, I don't yet see anything that suggests it's impossible to form it under milder conditions... it's an open question IMHO.
|
If it is an open question you should be able to cite literature that agrees with you.
(I will point you to an excellent Rand paper that provides a good survey of the subject, though since it is from 1975 it does not have the latest
calculations or experimental results:
http://www.rand.org/content/dam/rand/pubs/reports/2006/R2056...)
[Edited on 30-12-2014 by careysub]
[Edited on 30-12-2014 by careysub]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Okay careysub, you are right, it is impossible for metallic hydrogen to be made at STP.
Thanks for pointing out that I suffer from an illusionary superiority complex. I must also apologise for questioning 80 years of quantum chemistry by
such greats as Wigner, Metropolis, Bethe and Feynman.
Sadly, this is not the first time I've done this either. Just recently, in another thread of mine, I questioned the claims that hydrogen could be
harvested from the atmosphere to power fuel cells from the press releases of the research by another Nobel laureate.
Thank you for enlightening me about the "Dunning–Kruger effect"... and the acronym BOTE.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
dH. Thanks for raising this. It has been a good read. And I like your title. Tis a small step from imagination to speculation anyway. Good science is
about raising good questions. The fact hat the lit reveals that the question has already been answered does not detract from the validity of the
discussion.
@careysub. Love your posts.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks j_sum1 (flattered).
Back to the topic at hand. I alluded to earlier that the other strategy could be to try to make metallic hydrogen inside single-walled carbon
nanotubes to maintain a squeeze. This could in principle be done electrolytically, though I cannot dream up any practical cell for doing so that would
prevent reduction on the outside of the tubes and inside instead. Yes one could try to coat the nanotubes in an insulator... but how exactly? Not that
simple.
Another option is to fill the carbon nanotubes with something like lithium hydride, perhaps simply by melting the two together, then washing the
exterior LiH off with a saturated solution of LiOH in alcohol and then water. Hopefully, the inside ends of the carbon nanotubes would form LiOH plugs
and prevent further reaction internally.
There is precedence for filling carbon nanotubes as reviewed here [1].
The reasoning here is that LiH, in a weird way and in spite being an insulator, is close to metallic hydrogen in the sense that hydrogen is packed
very densely, except one would need to swap out the lithium for more hydrogen.
Now it's already known that hydrogen cations can diffuse through graphene [2], so I had already speculated that protons should also be able to diffuse
through single-walled carbon nanotubes (SW-CNTs) since they're just graphene sheets rolled up once.
The only problem... and it's a big one... is getting the lithium cations to diffuse out. I have a sneaky suspicion that unlike hydrogen cations,
lithium cations wouldn't be able to do this. However, if they could by some small miracle, then one would *simple* need to wash the LiH filled SW-CNTs
with a strong acid to exchange the lithium cations for hydrogen. The inside would become filled with H+ and H- at high density and possibly this
could form metallic hydrogen as the electrons redistribute.
I had read somewhere that very hot LiH (just below it's melting point) is a fast ion conductor (wiki?), so possibly the Li+ could diffuse through the
'cap ends' and out if the cap ends permit lithium conduction. But then this would have to be done by immersing the LiH filled SW-CNTs in molten
lithium hydroxide or a lower melting eutectic saturated with LiOH to protect the cap ends?
Again, the big problem with this is that I don't think lithium cations can diffuse through SW-CNTs like protons possibly could? But diffusion out the
ends may be possible atr elevated temperatures.
Below is a very bad drawing for this hypothetical idea 
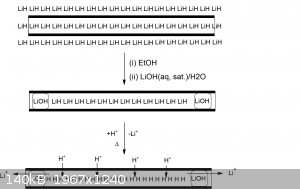
References
[1] Monthioux, M. (2002). Filling single-wall carbon nanotubes. Carbon. 40 (10). p. 1809–1823.
[2] S. Hu et al. (2014). Proton transport through one-atom-thick crystals [letter to the editor]. Nature. 516. p. 227–230.
CORRECTION: Wrote "lithium hydrogen" above where I meant lithium hydroxide... now corrected.
[Edited on 31-12-2014 by deltaH]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
There has been some interesting (mostly theoretical) work on hydrogen and carbon nanotubes. This one is discusses the transformation of SWCNTs by
decoration with hydrogen atom, thus metallizing the tube itself:
"Effects of hydrogen adsorption on single wall carbon nanotubes: Metallic hydrogen decoration" by O. Gülseren, T. Yildirim, S. Ciraci
http://arxiv.org/abs/cond-mat/0208510
The interaction of hydrogen bonding with the carbon directly must be an important effect.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks careysub, this is indeed an interesting paper... a kind of carbon nanotube equivalent of graphane. I'll enjoy
working through it.
I was thinking a while ago that packing hydrogen tightly on the inside of nanotubes might ease the metal transition, or it could have the opposite
effect. Generally speaking, electronic effects of surfaces significantly affect at least a couple of atomic layers above it (diminishing rapidly as
you move away)... so internally there would be significant effect on a significant percentage of the hydrogen present because they are only a few
layers away from the nanotube shell. In essence, the metallic hydrogen could become a partial hybrid between the properties of purely metallic
hydrogen and the carbon nanotube.
Sigh, I wish I had access to molecular modelling software, this would make for a fascinating study!
*****
You know, I'm still uncertain of the third step in my sequence. I know what I want to achieve there: hydrogen cations to diffuse in and lithium
cations to diffuse out gradually converting LiH to H('x').
Classically, something like this in a normal experiment would be done with an acid, BUT you don't want to dissolve the lithium hydroxide caps.
So my answer to this is use LiOH as the 'acid', provided that LiH is basic enough to 'pull' that second proton off.
On a macro scale, would this reaction proceed:
LiOH(l) + LiH(s) => Li2O(s) + H2(g) ????
If so, then there is a chance for using this reaction to strip the lithium from inside the nanotube and keep the end caps preserved.
[Edited on 31-12-2014 by deltaH]
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
You have access to hydrogen and single walled carbon nanotubes. Maybe the 'real thing' would be a good enough consolation prize?!
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I do? That's news to me  What do you mean forgottenpassword? What do you mean forgottenpassword?
To even attempt this, one would need a tube furnace that can be flushed with an inert gas. Maybe some day I might have such toys, certainly not now

|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
Do you not?! I can give you a link to someone selling single walled carbon nanotubes on ebay, if you like. I've even seen a thread on here where
someone was giving them away for free. Hydrogen you can make for yourself.
|
|
|
| Pages:
1
2 |
|