| Pages:
1
2
3 |
menchaca
Hazard to Self
 
Posts: 80
Registered: 12-3-2003
Member Is Offline
Mood: No Mood
|
|
copper fulminate
Is the copper fulminate prepred in the same way that Hg or silver fulminate?
just absolut ethanol must be used?
or common 96% can be used?
thanks again!!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Unfortunately no!
Hg(ONC)2 + 2Na --> 2NaONC + Hg
Hg(ONC)2 + Ca --> Ca(ONC)2 + Hg
Ca(ONC)2 + CuSO4 --> Cu(ONC)2 + CaSO4 (s)
Metathesis is the way to go!
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Reaction with Na or Ca isnt metathesis, its redox. Nor would I be happy actually doing this, what solvent? Not solid, cant be aq, would the
reduction destroy the fulminate in the process?
Digestion of silver or mercury fulminate with sodium chloride is supposed to produce a solution of sodium fulminate. Many soluable TM chlorides
should work as well here, but I'd expect silver to be much easier than mercry. I personally think that adding very dilute HCl to silver
fulminate would work for producing a solution of fulminic acid, but excess acid, too high a Ph would probably destroy it by hydrolysis. In the
specific case of making copper fulminate, copper powder will displace both silver and mercury from the corrisponding silver or mercury fulminate.
Keeping this all in solution is probably a good idea, some solid fulminates are supposed to be very unstable solids (relative to silver and mercury)
and Ive read alarming things about solid sodium fulminate.
If it were me, I would make silver fulminate, and keep it wet at all times and digest with the chloride of choice. This despite the large difference
in stability to physical shock of mercury fulminate. The amounts I play with I'm much more worried about the cumulative effect of toxic mercury
build up than blowing bits of me apart. Silver salts are slightly toxic.
[Edited on 10-4-2003 by Marvin]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Cu, Ag and Hg have the same electropositivity; thus none can be displaced by the other!
On the contrary a more reactive (reductive) metal like Na, Ca, Zn, Al would displace the Hg or Ag fulminate!
The process to pass from Hg(ONC)2 via NaONC to make Cu(ONC)2 that can't be made directly from Cu(NO3)2 + Ethanol + HNO3 (as Ag or Hg fulminates)
IS A METATHESIS!!!!!
Edit by Polverone: not so many mad faces!
[Edited on 10-4-2003 by Polverone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
shock and horror!
| Quote: | | Cu, Ag and Hg have the same electropositivity; thus none can be displaced by the other! |
I never thought that I would catch Philou Zrealone in error! It is shocking and amazing 
But Cu, Ag, and Hg certainly do not have the same electropositivity! Copper wire in silver nitrate solution soon becomes coated with silver crystals
as the more electropositive copper is oxidized and less electropositive silver cations reduced.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Ok you got me on the words!
I was refering on electropositivity as opposed to electronegativity!
Electronegativity of Hg, Ag, Cu is 1,9 and thus electropositivity is 2,1!
You spoke about electric potential and there it is true that Ag(+) is a stronger oxydiser than Cu(2+) and Cu(+) and Hg(2+) a little more than Ag(+)!
Thus Cu will displace Ag(+) and Hg(2+) salts and Ag will displace Hg(2+); but if the effect is strong with highly soluble nitrate salts it will be
another story for the very unsoluble Hg and Ag fulminates- read very slow and uncomplete --> USELESS!
The use of Zn, Al, Mg, Ca, Na will help a lot because potential difference is bigger what gives some drive force to the reaction and with a proper
choice, you will get a soluble fulminate salt that will allow nearly all metathesis one could think off!
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Mercury fulminate with fine copper powder works very well, though I cant remeber which book I read it in. It might well be mellor volume III, which I
dont own a copy of, so I cant check. Its not grindingly slow or incomplete to all intents and purposes, that isnt how redox works, which is one of
the few nice things about redox generally. Solubility of silver fulminate is about 1g/litre at room temp so I wouldnt call this highly insoluable
either.
If you are primarily concerned with the solubility, I dont see how a better reducing agent would be faster, its the concentration of the ions in the
solvent in contact with the surface area of the metal being oxidised that make the biggest difference here. Unless that is you are producing
secondary reducing agents like hydrogen which are partially solable in the medium and thus break the surface area problem.
Using sodium or calcium metal sounds downright dangerous, and I suspect they are strong enough to reduce the fulminate itself, even assuming a
suitable solvent can be found.
My idea about silver and sodium chloride wont work. Only half of the silver is precipitated according to one of my older books, becuase it forms a
silver fulminate/ sodium fulminate double salt. Same problem with potassium. Copper chloride may or may not work. I think it stands more chance.
The same method with mercury fulminate is supposed to work properly (one of the Faraday lectures I think), though the key word here is
'digest'.
Replacing the metal atom in mercury fulminate isnt automatically metathesis, this is the exchange of the radical componants of salts. If you add
copper powder for example, to produce copper fulminate and mercury metal, its not metathesis.
|
|
|
Boob Raider
Harmless

Posts: 33
Registered: 15-10-2002
Location: Canada
Member Is Offline
Mood: Picros
|
|
What about
Having a solution of Hg(ONC)2 in NH4OH and adding dilute NaOH solution in it. Wouldn't that ppt. out the Hg from the complex and leave NaONC in
or out of solution.
Actually, I think Hg(OH)2 will be soluble in NH4OH and NaONC won't. This is just a thought, may or may not work.
[Edited on 14-4-2003 by Boob Raider]
\"Go down in a Blaze of Glory\"
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I just wonder how soluble is Cu fulminate?
If it is unsoluble (what I think) it will coat the Hg fulminate grains very fast reducing the speed of reaction!Because Cu(2+) will have to pass the
Cu fulminate coating!
In all my books where fulminate is mentionned they speak about Na fulminate to be used for precipitation reactions of various fulminates; Cu included!
True that there is a mention about the Cu powder as a way to make it but no doubt purity and isolability is much easier when you start from a very
soluble Na fulminate!
Na or Ca methanolate?Would do the job!
I really think Na2S and CaS will get you somewhere since HgS is hell unsoluble (solubility is inferior to 1 molecule per liter!!!! Ks = 2*10e(-49))!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
PAS Smith has a reference from 1894 that uses dilute sodium amalgam to make sodium fulminate from mercury fulminate. This can be done and it sounds a
lot safer than I thought, so you got me there.
In a 'doh' moment I remebered fedoroff, and this is the most detailed information on copper fulminates.
The synth it mentions for Copper(I) fulminate is copper amalgam acting on silver fulminate in an atmosphere of hydrogen, I think copper turnings would
work. This is a water insuluable powder which is pretty much expected for a psuedohalogen copper(I) compound, Id have thought of this before, but
what I assumed we were trying to make was 'copper(II) fulminate', the fact copper metal reduces copper(II) ions to copper (I) should have
tipped me off about this, dumb dumb dumb.
Copper(II) fulminate isnt listed. Assuming this exists, Id expect it to be water soluable, but from what I'm reading this isnt whats is usually
refered to as 'copper fulminate'.
There are quite a few complexes that copper fulminates form, and this branch of chemistry seems to be a mess. In particular mixing sodium fulminate
with copper(I) chloride at RT produces copper(I) disodium fulminate as a ppt, and at 80C, sodium copper(I) fulminate. A copper(II) nitrite solution
acting on a sat solution of sodium copper(I) fulminate produces copper(II) dicopper(I) fulminate ppt. What with fulminate being a psudohalogen, I
should have furthur suspected this chemistry might be dominated by complexes.
What with the increased complexity, and problems using the less toxic silver compounds, I nolonger plan to try any of these at least in the near
future.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
There is also a mention about a trimeric fulminic acid --> fulminuric acid...some kind of cyanuric and isocyanuric acid isomer!
Cyanuric acid is (-C(OH)=N-)3
Isocyanuric acid is (-CO-NH-)3
Fulminuric acid is (-CH=N(O)-)3
or (-C=N(OH)-)3 where the carbon is trivalent (carbon is not always tetravalent ex C=O, R-NC (carbylamines)!
Thus we have trimerisation of the possible isomers of cyanic acid:
HO-C#N cyanic acid
HN=C=O isocyanic acid
H-C#N->O
HO-N=C
Fulminuric acid is unstable (but a little more than fulminic acid) and displays unsoluble explosive salts that are heat sensitive.
More stable and powerful than usual fulminates!

 
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I meant that fulminuric acid is more stable than fulminic acid!Thus less unstable and not "more" unstable as I wrote by inadvertancy
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
How sensitive is copper fulminate to friction, shock, and static? I'm considering making it in the future(once I get TNP and one other
nitroaromatic made). Is copper fulminate about the same as mercury fulminate or even more sensitive like silver fulminate is from what I understand. A
comparison to AP would be good to know. I would just consider making mercury fulminate but I don't have any mercury metal to use.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
heres the stuff on itfrom federoff volume 3
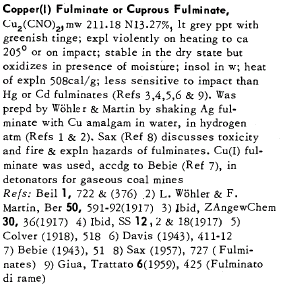
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
Thanks. It is good to know that about it being less sensitive to impact than mergury fulminate. I'll probably use the synth on powerlabs except
replacing Hg with Cu. I wonder if denatured alcohol would work in the synth or not.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
can't do that
IIRC, only silver and mercury fulminate can be made directly. All others are made by displacement reactions between silver or mercury fulminate and
different metals.
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
Always a bloody catch. Oh well, I'll just look into a more suitable primary then. Potassium picrate sounds a little weak but if it can at least
DDT in confinement it might be usable and isn't particularly sensitive. I'd prefer potassium styphnate but resorcinol to make styphnic acid
is hard to come by. Oh well, that's off topic anyway.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Any fulminate!
Quote:
"Fulminic acid is made by the action of nitric acid on alcohol."  
The magic phrase! And I've met it in about 10 websites about fulminic acid!
Now CF is easy, isn't it? CuO + fulm. acid.
Or a fulminate of any metal you can get an oxide of.
A word of warning: fulm. acid is explosive and about as toxic as HCN.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
wow, very nice, im gona have to try to verify.
anyone have a cluewhat the reaction would be?
ethyl nitrate is made via 70% HNO3/ethanol and distill off the ethyl nitrate, it is said that higher concentrations cause the HNO3 to do other
reactions.
but, we see in the Hg fulminate thing, we use 70% acid, i wonder whats going on, damn.
i just tok a small amunt of ethanol i used sucessfuly for fulminate production, put it in a test tube and added a little 70% HNO3 which i also used
inthe same synthisis, all i got was some warming. i smelled it and i think i could smell the ethyl nitrate, it has a damn low boiling point.
so, from what im lookingat here it says it must be somekind of reaction with the Hg nitrate and ethly nitrate.
perhaps high conc HNO3/ethanol makes fulminic acid?
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Just after I made that post I've written a balanced equation for the reaction:
3HNO3+2C2H5OH=>3HONC+CO2+6H2O
High conc. HNO3... I suppose so.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
i just found what i wanted, here we go
i wonder what makes the eaction happen at low conc with the synth of Hg fulminate.
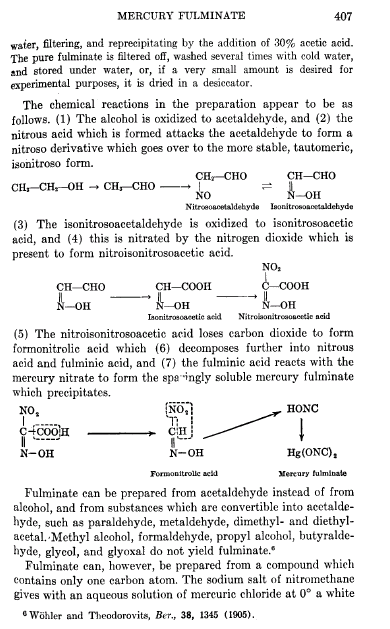
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
i just added some absolute ethanol to concentrated HNO3, very vigourous. then added a little Cu nitrate solution, no ppt.
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Any nitogen oxides observed? Then it was an oxidation, better reaction control needed. Gas evolution otherwise than NOx? Then the reaction probably
did happen. No bubbling? Then, I think, ethyl nitrate has been produced. No precipitate has been produced for good reason, it isn't produced when
you add H2O + CO2 to calcium nitrate, for example? What I mean is that fulminic acid is weak and the acidic environment doesn't allow the
precipitation to occur. Dilute solutions could help.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Its supposed to be an oxidation, you do get nitrogen oxides formed or you dont form any fulminic acid.
The problem is that fulminic acid is a partially oxidised product, and if left to its own devices will never occur in high concentration or yeild as
it will be destroyed as fast as its formed. This is why the synthesis of silver and mercury fulminates work, they are virtually insoluable in the
reaction mixture, so they ppt the fulminate ion protecting it from furthur oxidation. Its also the reason why virtually all other fulminates have to
be made through silver or mercury. They dont form highly insoluable salts, so the reaction fails.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Marvin:
What you say probably does happen with an excess of HNO3 (or maybe even with the stochiometric amount), but otherwise, (with an excess of alcohol) I
think, the partially reduced N atom would be integrated into the fulminic acid, which would be much less likely to be destroyed with an excess of
alcohol.
Madog:
"i just added some absolute ethanol to concentrated HNO3"
As I said above, It would be better to have excess ethanol in the reaction zone... or maybe even generally, so adding the HNO3 to alcohol would
probably work better. To test the results copper acetate would be better that strong-acid copper salts.
|
|
|
| Pages:
1
2
3 |