| Pages:
1
2 |
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Sniffity  | | Is there any way to confirm that hardware store HCl has impurities dissolved in it? What sort of impurities are we likely to find in hardware store
HCl, and why? |
Hardware store HCl will just about always be impure due to the nature of its production, and will generally appear slightly yellow in color. The color
is most likely due to aqueous iron salts and free chlorine, and possibly some organic compounds. Other possible impurities are sulfuric acid, nitric
acid, and chloride salts of sodium, calcium and lead. Really just depends on how it was produced.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I'd like some pure HCl(aq) for neutralizing a chlorate cell. Although the simple method presented by Robert Thompson is perfectly adequate, I'd like
to consider alternatives for getting it above 20% to reduce storage space.
I've thought of 2 ways by controlling temperature and pressure.
1. Just distill HCl from A to B under high pressure.
2.
a. connect 3 glass vessels A, B, C. Fill A with impure HCl (aq). Fill B with water.
b. quickly create a vacuum and heat the contents of A. This should cause the dissolved HCl to start evaporating. Not much will dissolve in B due to
the low pressure.
c. Without disturbing any seals, pour the now relatively depleted acid in A into C. Then close off the valve between A and C. This should still leave
almost all the gaseous HCl in A & B.
e. Now pump in air from the outside to reduce the partial pressure of HCl, causing it to dissolve in B.
#1 should be simple, but would it be too slow since the high pressure will tend to keep HCl in solution? Also, what pressure do I need to get say 30%?
Is #2 worth pursuing? My question is how much HCl can I cause to come out of solution using low pressure and high temperature? It seems if I can make
> 80% of the original 30% come out, then it would be worth it.
[Edited on 2-2-2015 by UncleJoe1985]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by UncleJoe1985  | I'd like some pure HCl(aq) for neutralizing a chlorate cell. Although the simple method presented by Robert Thompson is perfectly adequate, I'd like
to consider alternatives for getting it above 20% to reduce storage space.
|
It's nice of you to include a reference but 'Robert Thompson' won't mean that much to most here (myself included).
To reduce storage space? Blimey, just how much HCl solution are you planning to store? 
And the contents of A to start boiling, of course. At the top of Mount Everest water boils at 70 C.
I believe to prepare HCl > 20 % dissolving a known amount of pure HCl gas in the required amount of water is the only practical way.
[Edited on 2-2-2015 by blogfast25]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Looking at the WIKI page for HCL (http://en.wikipedia.org/wiki/Hydrochloric_acid) there is a boiling point chart that indicates BP's lower than that of water.
I may be wrong so correction may be needed but I believe that a fractional distillation rig can be used targeting specific temps bellow 100* C at
atmospheric pressures.
As you stated heating, and collecting the vapor should be the same as the room temp vapor transfer method . I can see that saving some time.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zombie  | I may be wrong so correction may be needed but I believe that a fractional distillation rig can be used targeting specific temps bellow 100* C at
atmospheric pressures.
|
You're aware of the (approx.) 20 w% HCl/water azeotrope, right?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Yes sir. There is a point of diminishing return I believe it is 35-37% concentration. I am simply looking at the purity aspect more than the
concentration.
UncleLoe is asking from a starting point of 20%, and I assume OTC purity. I don't think much "storage space will be gained but "pure" was my focus.
The Thompson vid. (method) is linked on page one of this thread.
[Edited on 2-2-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I believe that he is referring to this video.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zombie  | Yes sir. There is a point of diminishing return I believe it is 35-37% concentration.
The Thompson vid. (method) is linked on page one of this thread.
|
Now what did I tell you about expressing yourself concisely? 
At STP, 35 w% (approx.) is simply the solubility limit of HCl in water. No need for flowery language.
Thanks for the link reminder (washing egg off face now).
[Edited on 2-2-2015 by blogfast25]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Standard conditions for temperature, and pressure. Got it
Thanks!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Nevermind about thinking it can save space. It was just a familiar feeling since here in the San Francisco bay area, space is kind of at a premium.
For neutralizing a chlorate cell, I only need small, but pure amounts of HCl. For making NH4ClO4, I would need a lot of HCl and NH3 (hopefully neither
needs to be pure).
| Quote: | | a fractional distillation rig can be used |
Have you done this? because I doubt it. I found this phase diagram,
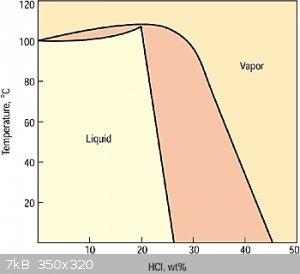
but I don't think it can be used much since something being distilled isn't a system in equilibrium.
But intuitively, the initial vapor will be very high in HCl, probably over 45% by weight. At that percentage, no amount of chilling is going to make
it a liquid. It's all going to escape into the air.
Even if the HCl in the vapor is lower at say 33% and 80C, after chilling it to 20C,
gas weight(both HCl and H2O) = 47%, HCl in gas weight = 47% * 42% = 20%
liquid weight = 53%, HCl in liquid weight = 53% * 25% = 13%
25% HCl is pretty good, but overall that's only capturing 13% / 33% = 39% of the HCl from the initial hot vapor.
| Quote: | | collecting the vapor should be the same as the room temp vapor transfer method . I can see that saving some time |
No, my main purpose isn't to save time. It's to create a concentration significantly larger than the 15% the room temperature method achieves.
I'm still don't have a chart for how pressure can increase the % of dissolved HCl.
I also find it puzzling that according to the chart, for 31% HCl to be at equilibrium at 20C, 35% of the mass would have to be vapor! For a 1 gallon
jug that would be 860 liters of vapor (at STP)! Clearly, that won't fit inside that jug. That would explain why when I unscrew the cap, I hear a fizz
and why the air stinks up so fast.
So clearly, the 31% HCl isn't at equilibrium. How did it get so strong? Was it due to massive pressurization during manufacture?
[Edited on 3-2-2015 by UncleJoe1985]
[Edited on 3-2-2015 by UncleJoe1985]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote:
"I may be wrong so correction may be needed but I believe that a fractional distillation rig can be used targeting specific temps bellow 100* C at
atmospheric pressures."
That was the entire statement.
No I have not attempted to distill Hydrocloric Acid to concentrate it. I did however ask on another thread about evaporating same, and was informed
that evaporation can be achieved to reach the STP 20% concentration. Again however the point of diminishing return...
http://www.sciencemadness.org/talk/viewthread.php?tid=17638
(2nd page near the end of the thread)
The only other option I can think of would be to bubble Hydrogen Chloride gas thru your existing HCL to the point of saturation.
I'm obviously still learning, and attempting to help or stimulate ideas where I can.
[Edited on 4-2-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I only see this dirtied 36% acid with cloudy soaps to prevent its use as a reagent but I had found a solution.
by adding tiny amounts of alum no more than a pinch help precipitate that cloudy polymer no distillation required.
however the acid will have a tiny amount of alum the amount used is really small but theres no other way than distilling to get it at higher
concentration.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Never have I ever used any grease on glass joints and had zero issues. Aspirator vacuum pump holds vacuum for a long after I've stopped it. I never
store anything with joints attached though, to avoid jamming. I had once almost a breakdown with one joint this way, and have scrupulously keeping
them apart since.
Teflon sheets 0.5-1mm thick and plumbers tape are excellent sealants for any non-glass joints. I have a couple of flange joints on SS equipment and
I've made teflon gaskets that work like a charm.
|
|
|
Frankenshtein
Harmless

Posts: 25
Registered: 20-11-2018
Location: ahead
Member Is Offline
Mood: oligomerized
|
|
I came here to post the Robert Thompson video but I see that I'm too late.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by HgDinis25  | It's a matter of price. Most hardware HCl is dirt cheap but either has some contamination in it or isn't concentrated enough. In my case, just buying
the HCl is much more rentable than making it from Sulfuric Acid and Chloride salts.
If you can buy 33/37% HCl but it has impurities in it, simple distillation should remove them and leave you with fairly pure Hydrochloric Acid.
On the other hand, if your store only sells diluted stuff, you may want to concentrate it. HCl and water form an azeotrope that boils at 110ºC and
leaves you with 20% concentrated stuff. If you need more concentration, adding Sulfuric Acid and bubbling the resultant HCl gas may be an option...
|
Distillation is a crappy idea.an hr or more of boiling acid sounds terrible.everyrhing U use around it will probably rust. To concentrate or purify
just put some hcl in a coke bottle drop in some calcium chloride or Al foil and lead fumes thru half as much clean water as hcl used.instant conc
clean hcl.al foil is better and quicker than damprid.
Over and done with in a few minutes.no need to distill.the HCl goes from one bottle to the other so easily
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by mysteriusbhoice  | I only see this dirtied 36% acid with cloudy soaps to prevent its use as a reagent but I had found a solution.
by adding tiny amounts of alum no more than a pinch help precipitate that cloudy polymer no distillation required.
however the acid will have a tiny amount of alum the amount used is really small but theres no other way than distilling to get it at higher
concentration. |
Drop a big piece of Al foil and lead fumes thru distilled water.easy.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
However, it is possible to purify crude hydrochloric acid for other purposes using the two-container technique.
http://www.sciencemadness.org/smwiki/index.php/Hydrochloric_...
I'm glad to see that this was added to the wiki
This is the only video on it
https://m.youtube.com/watch?v=jv1Ms6Subg4
The process takes a week or two to finish, and the resulting reagent-grade acid is about 5 molar rather than the 12 molar typical of commercial
concentrated hydrochloric acid. Still, it's extremely pure and concentrated enough for most purposes.
if it was in a reduced pressure environment (small vaccum);it would speed the process along faster
[Edited on 24-10-2020 by symboom]
[Edited on 24-10-2020 by symboom]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
How much did the hw store HCl cost?
It determines a bit how much of an option it is to do it yourself from scratch.
If sodium bisulfate or sulfuric acid is available for cheap, then probably it is. Hydrochloric acid and hydrogen peroxide are two products that
command an exceedingly high price per mol of reagent for consumer market. For example, I had otc 3% H2O2 available for 12€ per liter, which
translates to same per mol. 35% costs the same, it just needs one trick to buy it, being almost 12 times cheaper per mol.
Here, pool stores sell 35% HCl in canisters. They are cheap, like 20-30eur per can of 30 liters, but the shipping quickly becomes an issue and doubles
the price.
You could check how much it would cost to use calcium chloride to desiccate the HCl and dissolve the gas in new solvent?
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Small amounts of hydrogen chloride for laboratory use can be generated in an HCl generator by dehydrating hydrochloric acid with either sulfuric acid
or anhydrous calcium chloride.
That's use full so if you add solid calcium chloride it will push the hydrogen chloride out of solution in a closed system in into another container
with distilled water.
Thats a great improvement than just waiting a week
_____
This reminds me of concentrating nitric acid procedure with
Magnesium nitrate
The principal use is as a dehydrating agent in the preparation of concentrated nitric acid
[Edited on 24-10-2020 by symboom]
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Where I am, 8.5% HCl in tile cleaner cost $1 per litre.
I just boil half a portion away (mostly water) to get to 15-16%, then add 10% by weight of anhydrous CaCl2 and do a simple distillation.
I managed to get a clean clear 24-25% HCl (by density).
I supposed you can get a higher concentration by adding 20% or 25% CaCl2, but I don't need a higher concentration nor want to store one.
[Edited on 24-10-2020 by artemov]
|
|
|
| Pages:
1
2 |