aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ammonia Lamp
In researching possible routes to Nitric acid, i came across a few references using Ammonia as a starting material.
Lacking any Platinum, i tried a 3mm hoop of 15m copper pipe attached to a length of steel wire, heated to redness, then suspended above some 30%
ammonia solution in a 500ml vacuum flask.
The copper hoop remained at red heat, so obviously some highly exothermic reaction was going on.
Trying the same process in a normal erlenmeyer of the same volume caused the glowing Copper hoop to cool much more rapidly (10 seconds) compared to
the vac flask (several minutes).
I suspect that Air was being sucked in via the vac tube on the vac flask.
Catalytic Oxidation of the Ammonia appears to be the answer to the Process, however i am at a loss as to what the Products may be.
Any ideas ?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Nitrogen gas, possibly nitrogen oxides.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The flask remained clear at all times, so no NO2 then.
Doh !
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Probably nitrogen and water, with a tiny amount of nitrous oxide and negligible amounts of hydrazine.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Perhaps lesser known is that N2O (so called Laughing Gas) and access to a microwave can be employed to form NO. See opening discussion at http://www.sciencemadness.org/talk/viewthread.php?tid=34429 .
Here is an edited version with a nitrate only focus:
Quote: Originally posted by AJKOER  | I have performed multiple runs of a new path to nitrate based on microwave treatment of an aqueous mixture of HOCl (preparation discussed below),
8.25% chlorine bleach (NaClO) and CaCl2, that was infused with N2O gas (source is an 8 gram of N2O pressurized gas cartridge designed for use in a
cream whipper which was employed here) and oxygen enriched air. A small amount of chlorate is also formed which can subsequently be greatly increased
via photolysis using the reported photocatalytic properties of the created nitrate. Actual portions employed in a particular run are detailed below
along with precautions and photos.
First, some theoretical background to provide understanding, promote safety and good yield.
One interesting paper is "Treatment of N2O impulsed microwave torch discharge " by M.Jasinski, et. al., April 2004 . To quote: "Results of using a
moderate–power (several hundred Watts) pulsed microwave torch plasma (MTP) to the conversion of atmospheric-pressure nitrous oxide (N2O) into
nitrogen oxides( NO, NO2 and N2O4) are presented." with a claimed efficiency between 70% to 90% depending on the power of the microwave. Link: https://www.google.com/url?sa=t&source=web&rct=j&...
This possible conversion of N2O is also cited in this source, "Focus on Hazardous Materials Research", edited by Leonora G. Mason, to quote : "In our
previous study using microwave discharge, N2O could be efficiently decomposed into N2 and O2 at atomospheric pressure [8]. However, when N2O is
diluted in N2/O2 mixtures was decomposed by microwave discharge, a large amount of NO was emitted due to fast reactions as N(2D,2P) + O2 --> NO +
O link: http://books.google.com/books?id=8MXX01Qw_G0C&pg=PA144&a...
So, for the purpose of constructing a net reaction for the gas phase in the radiolysis of N2O diluted in N2 and a O2 enriched mixture in a microwave,
I will assume, as an undoubted simplification, a reaction sequence that has one of the larger associated oxygen (which is supplied pre-run) and
nitrogen demands:
1/2 N2 + pulse radiation ---> N(2D,2P)
N(2D,2P) + O2 --> NO + O
O + N2O ---> 2 NO
Simplied net reaction ignoring possible benefical formation of any NO2 and several other intermediate nitrogen and oxygen species:
1/2 N2 + O2 + N2O --pulse radiation--> 3 NO
With shaking of the pulsed radiated vessel, a reaction with Hypochlorous acid:
2 NO + 3 HOCl + H2O --> 3 HCl + 2 HNO3
where the scrubbing of the NO is best accomplished between a pH of 4 to 7, and not more alkaline (see "Oxidation of Nitric Oxide in Two Stage
Chemical Scrubbers Using DC Corona Discharge" available at https://www.google.com/url?sa=t&source=web&rct=j&...
.......
Next, apparently N2O in an aqueous setting has somewhat different chemistry on irradiation. Per this source, "Chemistry of Ozone in Water and
Wastewater Treatment: From Basic Principles to Applications", on page 229, the author Clemens Sonntag cites the following reaction involving a
solvated electron (actually, rescaled by 4, link: http://books.google.com/books?id=Om_TKidEjToC&pg=PA229&a... ):
4 e-(aq) + 4 N2O + 4 H2O ---> 4 ·OH + 4 N2 + 4 OH-
where there is no nitrate formation......
With shaking, the hydrolysis of the NO2:
4 NO2 + 2 H2O --> 2 HNO2 + 2 HNO3
and, with further addition of HOCl:
2 HOCl + 2 HNO2 --> 2 HCl + 2 HNO3
Also:
4 NaOH + 4 HNO3 = 4 NaNO3 + 4 H2O
.......
Future research, microwave a mixture of CO2 and N2O, in the presence of water and a substance providing an electric discharge, in a fashion similar to
US Patent 5,266,175, link http://www.google.com/patents/US5266175, where a mix of CH4 and CO2 in a microwave has been patented by Exxon as a path to syngas (CO + H2), see
also discussion at http://www.wvcoal.com/research-development/spain-co2-ch4-hyd... . I have previously reported on SM sparking in the microwave reaction between N2O
and NaOH (see , http://www.sciencemadness.org/talk/viewthread.php?tid=32334 ) and more recently, to a limited extent, I witnessed it between aqueous NaHCO3 and
N2O. Now, as the action of uv light on CO2 has been reported to produce Singlet oxygen (to quote a source "We present a first principles study of the
carbon dioxide (CO2) photo dissociation process in the 150-to 210-nm wavelength range, with emphasis on photolysis below the carbon monoxide + O(1D)
singlet channel threshold at∼167nm.", link: https://www.google.com/url?sa=t&source=web&rct=j&... ), I suspect it may be one of the caustive agent in the patented microwave
synthesis. In the current context, the reaction of Singlet oxygen and N2O is known to form NO especially on cooling (see http://scitation.aip.org/content/aip/journal/jcp/78/2/10.106... ).
----------------------------
Sample Run Procedure (picture below of results from half of reaction, with the other half in a photolysis run):
1. Combine 154 ml of 5% vinegar and 11 g (around 5 ml) of NaHCO3 to form CO2 gas in a 3 liter vessel. Drain fluid leaving just CO2 gas.
2. Combine 209 ml of 8.25% NaOCl with 13.1 g (around 6.2 ml) of CaCl2 in a new vessel. Stir.
3. Add (2) to (1) and shake forming a bright white suspension of CaCO3. Cool to allow CaCO3 to settle, and pour out just the clear HOCl.
4. Infused the HOCl in (3) with an 8 g cartridge of N2O in a cream whipper. Shake to aid in the N2O infusion, which is limited in water.
5. In a 3 liter vessel (I used a 3 liter Poland Spring clear plastic water bottle that can standup in a microwave oven) react 28.7 ml of 3% H2O2 with
20.3 ml of 8.25% NaOCl. Drain all of the liquid leaving air enriched with O2 gas. Compress the vessel by 1/3 to allow for thermal expansion.
6. Place about HALF the contents of the infused HOCl from Step (4) into the enriched oxygen vessel of Step (5).
7. Radiate the vessel in a microwave for between 4 to 7 seconds only until the vessel is fully inflated. Remove the vessel, shake and cool by
inserting into an ice water bath as needed to implode the vessel. Caution: Do not attempt to microwave the fully expanded vessel since if the N2O
undergoes a thermal decomposition (sounds like a loud knock and the vessel acts if it was struck), the sudden rapid increase in volume from 2 N2O
---> 2 N2 + O2, coud cause a pressure eruption of the vessel.
8. Repeat Step (7) ten times or until shaking the vessel after a microwave treatment produces little compression.... |
Note, one does not have to scrub the NO with HOCl, one could employ NaClO (chlorine bleach) pH adjusted to neutral or a little acidic avoiding the use
of organic acids (like acetic, critic, oxalic,..), a peroxide or possibly oxygen gas. Also, I have not as of yet confirmed that microwaving a mixture
of CO2 and N2O, in the presence of water and a substance providing an electric discharge (perhaps Aluminum foil) in a fashion similar to US Patent
5,266,175, link http://www.google.com/patents/US5266175, safely forms CO and NO primarily (see http://en.m.wikipedia.org/wiki/Reaction_mechanism for possible additional reaction paths, and in the event of any hydroxyl radical creation with
irradition, I have not rule out also the possibility of a minute quantity of a cyanide).
FYI, N2O cartridges are widely sold (in the same form of small CO2 cartridges) and are relatively inexpensive. Unfortunately, Nitrous oxide has been
the subject of some abuse (as a party gas).
[Edited on 12-1-2015 by AJKOER]
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
The youtube channel Hegelrast tried this but with a Platinum wire.
https://www.youtube.com/watch?v=CnhAnhK7U18
The catalized oxidation of ammonia goes as follows:
4 NH3 + 5 O2 → 4 NO + 6 H2O
The NO formed should quickly form NO2. However, just like in Hegelrast video, the quantity of ammonia that oxidizes and thus the quantity of NO2
formed are quite low. I suspect that's the reason you don't see beautiful brown gas coming out of your vacuum flask.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Heterogeneous catalysis
Copper is a poor catalyst for the oxidation of ammonia compared to platinum, but there's plenty of room for experimentation and trying to improve it.
For heterogeneous catalysts, specific surface area is the name of the game. The catalysed chemical reactions only happens on the surface of the metal
and the bulk metal beneath is essentially wasted. This is why metals employed for heterogeneous catalysts are usually used as nanoparticles, so that
the ratio of surface atoms to total atoms in the particle is highest and so the catalyst is most active. This ratio is called the dispersion of the metal in the catalyst... the higher the dispersion, the more active the catalyst (usually).
Solutions of nano-copper are easy to make using ascorbic acid (vitamin C) as reducing agent and a copper salt. For example, see here. I'd avoid copper sulfate as sulfur is a notorious 'poison' for heterogeneous catalysts. Chlorides can sometimes speed up sintering (see
later), chlorides can be problematic for other reasons, but unless you have copper acetate or nitrate, probably unavoidable.
Now the biggest problem with heterogeneous catalysts is a process called sintering, this is when small particles fuse together to form bigger ones. For metal catalysts, this basically destroys your massive surface area
gotten from employing nanoparticles for which you worked so hard for  A rule of
thumb is that sintering will occur from one-half of the material's melting point, referred to as the Tamman temperature. A rule of
thumb is that sintering will occur from one-half of the material's melting point, referred to as the Tamman temperature.
For copper, the melting point is 1084.62 °C, so you should worry about sintering from as low a 500°C. Now herein lies the problem. When the ammonia
oxidation works, the metal would glow white hot, so it's likely to rapidly die if used as is 
The way catalytic scientists solve this kind of problem is to deposit the metal nanoparticles onto an inert carrier with an extremely high melting
point and large specific surface area itself. Things like gamma alumina, fumed silica or titania. In your case, the easiest OTC clone would be to use
silica gel, conveniently available as kitty litter  (check label for this type,
ingredient should say silica gel). (check label for this type,
ingredient should say silica gel).
So you can follow the instructions of your choice to prepare copper nanoparticle solutions, e.g. the website I linked above or any of the multitudes
available online and in literature.
Then soak this solution into the silica gel and leave out in a warm dry place until all the solution has evaporated or boil it off with the silica
added (NB) if you're impatient. Silica should easily be able to hold 10% its own mass as copper nanoparticles, so you can use this figure for
targeting a loading for the metal (you know the concentration of the copper in your reduced solution, so you can use that to figure out how much
solution to add to a mass of silica).
If you're lucky, this catalyst might work much better than your copper ring for the ammonia oxidation, though it might deactivate (die) rather
quickly, but even so, since it would be really cheap and you could prepare lots, no worries right? 
There are other tricks for heterogeneous catalysts, like using 'promoters', substances added in small amounts (a couple weight percent) to increase
activity even further.
[Edited on 12-1-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Forgot to mention something very important... you don't need to pre-reduce your copper before impregnating (depositing) the nanoparticles on the
silica support. You can simply impregnate the silica gel with a copper nitrate solution or acetate and dry. This will decompose to black copper oxide
nanoparticles on the silica with some mild heating (which you will do on a flame before lowering into the ammonia flask). Then when lowered into the
ammonia and while red hot, this should rapidly reduce to copper anyhow
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cool !
I have copper acetate and silica gel (dessicant stuff).
Will give it a try.
Thanks deltaH !
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Dude I found the procedure in some old book. Pt is supposed to convert it to Nitric Acid, then Ammonium Nitrate by neutralization once the vapour
reaches the Ammonia. I stumbled across a few lengths of Pt wire (literally) so I guess I could give it a try and report back..
[Edited on 13-1-2015 by Hawkguy]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Break a beaker aga!
While hypothetical/experimental catalysts are a notoriously difficult nut to crack, it's so much fun. One might be tempted think if cheaper catalysts
could do this, why does industry still use mostly platinum mesh for this reaction? The answer lies in chemical engineering, a very large industrial
reactor needs to run for several months on end (at the very least) or preferably a couple of years. While some catalysts that deactivate by surface
poisoning can be regenerated internally by clever reactor design, those that deactivate by sintering cannot and have to be taken offline, removed and
refilled.
Platinum is a very high melting and inert metal that is so active for this reaction that the tiny surface area of a solid mesh is sufficient for it to
work... this means the mesh can go on for a very long time without losing activity and that's the bottom line.
For us, if we could come up with a catalyst that lasts several minutes at a time or ideally a couple of hours, that would well good enough to make
small batched of nitric acid. For an industrial catalyst, this would be a total failure, but not for us!
Besides for impregnating silica with copper acetate, you may want to try silver nitrate to form a silver catalyst. There are other metal alternatives
as well...
In the end of the day, the yield of NO might be too low to be usable though, but positive thinking... 
[Edited on 13-1-2015 by deltaH]
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
I looked up on google "catalytic oxidation of ammonia with copper" which yield some interesting things. According to this link
| Quote: | When a heated copper coil is suspended over concentrated ammonia, it catalyzes the oxidation of ammonia to NO2 releasing more heat. The added heat
continues to heat the copper wire to its melting point.
|
That website also has references which could be interesting.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
This is great HeYBrO, it's nice to see that copper makes at least some NO! That's very encouraging, now to get the catalyst right...
Aga, if you setup an apparatus like this:
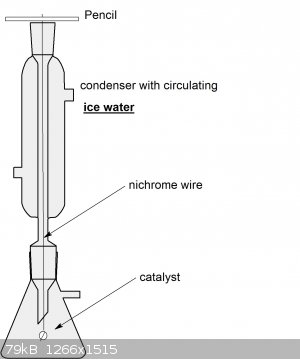
...you might be able to gradually convert your ammonia solution into a solution of ammonium nitrate. Add a drop of pH indicator, the solution should
go from very basic to slightly acidic and can be a visual cue that it's working.
Description of the apparatus:
You use a flask with a side arm, like your vacuum flask, so that air can be sucked in. You hold your heterogeneous catalyst as a bead with some
nichrome wire (can handle high temperatures and shouldn't corrode quickly). The bead is placed just below the level of a condenser inlet so that the
hot gases rise up it and fresh air is sucked in from the side arm. The condenser should be cooled from recirculating water in an ice slurry bucket by
means of a small fountain pump.
The way this is supposed to hypothetically work:
In the condenser, nitric oxide will react with oxygen to form nitrogen dioxide which condenses on the walls of the cold condenser. It drips back into
solution where it can react with water to form nitric acid and more nitric oxide goes up again. The nitric acid is immediately neutralised by the
ammonia, this generates heat and helps to evaporate more ammonia up to react on your catalyst.
[Edited on 13-1-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Now that I think about it, you might want to try nichrome wire on its own as a catalyst for this reaction  Nickel is a good hydrogenation catalyst and chromium is a good oxidation catalyst
and nichrome wire can handle very high temperatures! Nickel is a good hydrogenation catalyst and chromium is a good oxidation catalyst
and nichrome wire can handle very high temperatures!
It's easily ripped out of old hair dryers as a neat coil that would be perfect shape for the reaction, just dangle the coil vertically. Bonus, you can
experiment with heating it electrically!!! Perhaps at high temperature it works better and so making it white hot with a current might help the
reaction?
[Edited on 13-1-2015 by deltaH]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
this can be done
https://www.youtube.com/watch?v=1V0rIo8c7qc
http://upload.wikimedia.org/wikipedia/commons/b/b6/D%C3%B6be...
instead of Zn in H2SO4 ,any ammonium salt can be put in a tin box with holes and dipped into NaOH
[Edited on 13-1-2015 by CuReUS]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Aaah... chemistry porn 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Looks good ! I'll give it a go.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I came across an interesting comment on page 655 of "Water Chemistry: An Introduction to the Chemistry of Natural and Engineered ..." by Patrick
Brezonik, William Arnold. Link https://books.google.com/books?id=ltBoAgAAQBAJ&pg=PA655&...
, the author notes that Singlet oxygen can oxidize NH3 to nitrite and nitrate.
Now with respect to Singlet oxygen and my prior thread on the subject (link: http://www.sciencemadness.org/talk/viewthread.php?tid=31729 ), it is already a very cool experiment, and I would add the following comment by
Woelen:
So, upon introducing ammonia to a chamber of Singlet oxygen with a small amount of water/water vapor, I would hope for the production of a white
smoke of ammonium nitrite and nitrate.
--------------------------------------------------------------
There may also be a path using home ozone generators, as per Wikipedia on Ozone, to quote:
"Ozone does not react with ammonium salts, but it oxidizes ammonia to ammonium nitrate:
2 NH3 + 4 O3 → NH4NO3 + 4 O2 + H2O"
[Edited on 18-1-2015 by AJKOER]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The singlet oxygen is interesting AJKOER. I wonder what the yield of singlet oxygen is in such preparation methods?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
The absolute physical amount that is conventiently produced is undoubtedly low for the ammonia/singlet oxygen route for most of us.
However, this may not be a bad thing as half the product is, after all, a fine dry powdered NH4NO2, which to quote Wikipedia (link: http://en.m.wikipedia.org/wiki/NH4NO2
"Ammonium nitrite may explode at a temperature of 60–70 °C,[2] and will decompose quicker when dissolved in a concentrated aqueous solution, than
in the form of a dry crystal"
and, as I believe it is considered a high explosive (and quite toxic, so avoid inhalation of particles), you should really only experiment with very
small amounts.
For the record, as the action of singlet oxygen and water forms H2O2, I view the suggested synthesis as possibly an roundabout H2O2 preparation path,
which is also cited by Wiki:
"It can also be prepared by oxidizing ammonia with ozone or hydrogen peroxide,.."
So not really a completely new idea, but interesting that at least one author confirms its possible formation as it apparently easily decomposes in
aqueous preparation routes per an old thread http://www.sciencemadness.org/talk/post.php?action=reply&... Quote: Originally posted by Formatik  | ......
Ammonium nitrite in the aqueous phase is not thermallfy stable enough to survive being entirely boiled down. In fact, its decomposition in the aqueous
boiling state by mixing aq. nitrite with an ammonium salt is an old method of preparing nitrogen gas.
Those were some good finds on forming it from hydrogen peroxide, but unfortunately I've not seen these qualitative experiments transformed into a
workable synthesis. If I remember right, Gmelin has some good information references on its preparation using sodium nitrite as the starting point.
|
[Edited on 18-1-2015 by AJKOER]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | In researching possible routes to Nitric acid, i came across a few references using Ammonia as a starting material.
Lacking any Platinum, i tried a 3mm hoop of 15m copper pipe attached to a length of steel wire, heated to redness, then suspended above some 30%
ammonia solution in a 500ml vacuum flask.
The copper hoop remained at red heat, so obviously some highly exothermic reaction was going on.
|
substitute urea for NH3 and see if it works
http://www.sciencemadness.org/talk/viewthread.php?tid=4951#p...
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
If I may, I have first hand experience on the matter.
It is true copper does yield some nitrogen oxides when ammonia is oxidized on it, however the yield is minimal. It can be also said that the 50$ cost
of a 2 inch length of platinum wire is well worth it, especially since it is almost indefinitely re-usable in an home lab setting. One may also need
to know that any excess ammonia in the vicinity of the catalyst will destroy all the yield by complete oxidation of the ammonia to nitrogen and water.
The ammonia must always be in serious air excess, however this lead to a reaction that cant sustain itself. This explains the use of extreme pressure
in industrial processes. However, it is to be known someone with a length of quartz tube can use extreme heat to balance the cooling effect of the
excess air on the catalyst.
Hope this help out.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Does anyone know if palladium will work as a catalyst?
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Cool thread. A long time ago I also used copper as a catalyst for this reaction, my results weren't very good as I had limited glassware at the time.
To solve the over-oxidation problem mentioned by plante1999 an excess of air can be used.
A simple setup could be used, where air is bubbled through a conc. ammonia solution and then lead into the catalyst tube.
If you don't have conc. ammonia try dropping water over a mixture of sodium nitrate, hydroxide and aluminum.
However hydrogen my be produced in this reaction as well, so take care if you try this one.
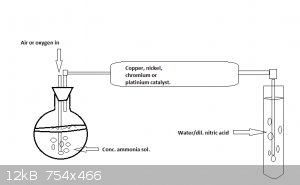 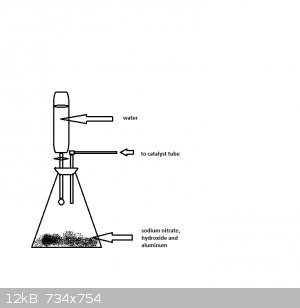
[Edited on 19-1-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|