chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
Butanole and other potential fuels from fermentation
As some have pointed out in another thread ( https://www.sciencemadness.org/whisper/viewthread.php?tid=61... ) it might be possible to get alcohols from fermentation of starch/corn/wheat etc.
. (for use as fuel etc.)
==> Someone even pointed out the possibility of using bakers yast for that .
Now: How efficient can that be done by amateur means, with readily available yasts ... ?
While searching on the net I have only found some quite new publications, where extra-efficient bacteriae have been used ... or where the
starch/grain/whatever had to be prepared by quite complicatred means ...
===============
If someone now, for example, would
==> boil some flour in an excess of water (to keep it liquid) ...
==> and then would let it cool to 25 [Celsius]
====> and then would add bakers yast ... 
What would happen ?
==> Would it ferment to any measurable degree ?
==> How much of the starch/cellulose could be used by the yast ?
==> Could one achieve a 100% conversion of the initial (poly)sugars to alcohols/ketones?
==> How much time would this take ?
[Edited on 28-1-2015 by chief3]
|
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
As I was reading around I found the following initial hints:
==> Moonshiners usually would want to reduce the starch with malt , beacuse they want mono- or disacharides ... that the yast ferments to
_ethanole_ for drinking .
Also those moonshiners do _not_ want any methanole or other alcohols ... which indeed may be formed by fermentation of starch ... ; eg. when producing
sake from rice there is a risk of producing methanole ....
This gives a point to the posts of @zobie (n the other thread), who says that yast can ferment any sugars ... ; it just isnt usually done because of
the reaons just mentioned ...
====
The second thing is: Hydrolysis might possibly be quite easily done by using eg. HCl ... ; would this be worth/necessary doing ... ?
[Edited on 28-1-2015 by chief3]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
acidify with citric acid to a pH of 4.5-5.0 and heat to around 80C for 40-45min. Don't boil the mash or you cook it instead of converting the
starches. Cool to 95F and add your yeast. They don't call it "sour mash" for nothing.
Just look at the process of making beer. Classic conversion of grain starch to alcohol.
As an added thought you should be able to skew your higher alcohol production just by controlling fermentation temperatures. I don't exactly remember
but I believe that higher fermentation temps tends to produce the higher alcohols.
[Edited on 29-1-2015 by hyfalcon]
[Edited on 29-1-2015 by hyfalcon]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Boiling starch does nothing for conversion. It simply releases the inner starch from the outer shell.
Both of these components of simple starch now have to be converted to simple sugars that can be fermented, and that can not be done with acids.
You need enzymes to convert. This link will save an hour of me two finger typing... http://en.wikibooks.org/wiki/Brewing/Mashing
Malted grains provide the enzymes, and sugars but either can be purchased/created separately. There is no need for use specific enzymes or sugars.
Acids or bases are used to speed the processes. Acids help release starch to it's components, and bases help keep yeast happy, and reproducing.
It's very simple stuff to accomplish, and nothing special is needed that can not be found around any household. A bucket, some starch or sugar, yeast
(there are many types but any will do), clean water, and perhaps a stove.
Distilling ETOH is as easy as making dinner. Doing it efficiently for a specific purpose takes simple specific methods. You can learn it all in a
couple hours if you use proven methods or spend a few months researching to design your own methods.
When you get to the point where you need to get a hands on approach for a column, feed system, re-boiler, condenser (reflux / collection) let me know.
I'll draw you up a quick CAD drawing, and component list.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
Allright ; suppose one gets some malt, as for making beer . Usually when buying the malt there will be a statement like : "Can be used to make X
liters beer" ... ; but those X liters wouldn't make it cost-effective .
==> Can I now use the malt to convert a much higher amount of eg. wheat-flour ?
==> Or will it do no conversion at all to additionally added flour ?
Since one would have to pay the ingredients ... a good rate of conversion would be essential ...
Just to give you some numbers which inspire me:
==> Wheat costs around 200 EUR/ton ...
==> Fuel in the EU costs 1.30 EUR/liter (much more expensive then in other countries)
==> Electricity costs 0.30 EUR/kWh ...
Which means: Readily usable Energy is very expensive over here ... , but: you can still get the raw ingredients like grains or coal etc. at
near-world-market prices ... and try to get towards the readily usable energies like fuel or electricity on your own ... ; still you would have to
calculate ...
but if it would eg. be possible to make 500 liters of ethanole from a ton of wheat ... you would have it at maybe 0.50 EUR/liter ... which would be
1/3 of the price you pay for gasoline ... ...
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Lets go thru some rough numbers together. I'm not gonna be 100% on the money but just to ball park this.
There is something in malt called Distatic power or DP https://www.google.com/search?q=Diatestic+power&oq=Diate...
This is the ability that a particular malt has to convert starches. From a distillers point of veiw Barley malt is able to convert many times more
starch than corn malt.
There are charts that rate the DP of most common malts, and malt can be made at home simply by germinating seed stock. You don't necessarily have to
buy malt, you sprout it.
Now it takes roughly 2-3 LBS of grain per gallon to make approx 8-10% abv mash. So 10 gallons of water, and 25ish LBS of grain will yield 1 gallon of
azeotrope ETOH.
Depending on conditions (yeast used / ambient temp.) you can ferment in anywhere from 3 - 10 days, and clear the product or settle it in another 2.
The process of settling allows you to reclaim your yeast. That's another good point. One packet of yeast can be cultured into a lifetime supply.
Bacarrdi has used the same strain,batch for 100+ years.
Anyway... Figure roughly 10gal mash equals 1 gallon of fuel, and it takes about 25 lbs of grain to get it.
You have to run a distillation at a set speed so 10 gal can be run in about an hour or less in a continuous feed 4" column.
IMHO Barley is best in grain, sugar beet is best in root crop, sugar cane is best in renewable overall.
Back to DP... You can use any sprouted grain to convert the starch from any other source. The ONLY issue is you need to heat/cook the starch at
specific temps to break the bonds, and that alone takes some of your energy. Not enough to break the bank tho. The same heat source that cooks the
starch can, and should be used to pre-heat your mash for injection into the column.
The fun part here should be designing your heat ex-changers off of your engine. Highest temp for the re-boiler, and lower temps for the pre
heating/cooking.
I might look at an oil bath kettle or a steam jacket kettle for the low temp aspects but don't let me influence that decision.
I'll attach some of the concentric column drawings I built just as reference...
I just found this boiler diagram as well. It might be of some use as it is designed as a "multi stage", and I'm certain it can be simplified as a pre
heater for the mash, as well as functioning as your boiler.
http://www.google.com/patents/US7967946
[Edited on 30-1-2015 by Zombie]
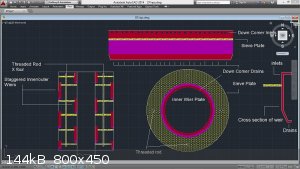 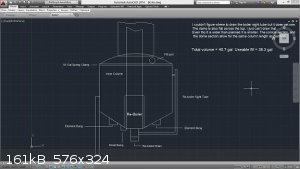 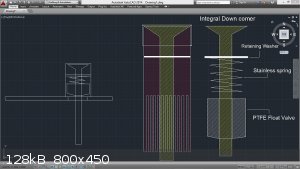
[Edited on 31-1-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Texium
|
Thread Moved
22-11-2023 at 19:11 |