| Pages:
1
..
4
5
6 |
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by deltaH  |
Well spotted blogfast, I missed that. That makes the corrected metal oxide values really small. Thanks!
|
Welcome.
Looks to me your Mg[Lin]<sub>2</sub> is already crosslinking during drying. That would make sense, of course.
Ever considered transesterifying that linseed oil to methyl esters? Separate off glycerol and then dry with desiccants? |
Transesterification would be great fun, but I don't believe transesterification would give as much odour control compared to saponification and would
detract from hardness, but thanks for the suggestion.
The thought of using the Mg[lin] 'as is' also crossed my mind, it's brittle and glassy, cracking and shattering on cooling very much like a glass.
I'm going a little overboard with isolating all these soaps and purifying them individually, but that's because I'm researching each's behavior.
Ultimately, when I come to a formulation recipe composition, it will probably be done in one step with the salting out/ion exchange, i.e. the
solution used will be a mixture salts, e.g. MgSO4, FeSO4, ZnCl2. So you will only need to dry once and all-in-all, it would be very amateur friendly.
Plus, while you have it hot on the pan at the end-of-drying, after a little cooling, that would be a good time to add the other ingredients and cast.
[Edited on 28-3-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
What I need now is a plasticiser for these brittle glassy linseed soaps, one option is using free fatty acids of linseed oil in my formulation. My
strategy will be to use a salting out/ion exchange of the Na[lin] with NaHSO4, which is obtainable cheaply as pool dry acid.
Aside: theoretically raw linseed oil could also work as a plasticiser, but I want all the glycerine OUT for odour control!
Does anyone know any OTC sources for zinc sulfate? It would be nice if that was available easily, because all the other metals have easily available
sulfates.
[Edited on 28-3-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Chemical conflagerations
Now that I have some brittle glassy dry Mg[lin]2, I could not resist performing a small and crude conflagration test.
I ground up roughly by volume (too small amounts for my scale) 1 part sulfur, 2 parts Mg[lin]2 and 4 parts NaNO3. This didn't burn very well in small
amounts, so I added a pinch of ferric nitrate nonahydrate and ground further. This formed a waxy moldable 'dough' of which I could light a small
finger-rolled bead. Photo's below (note the photo of the yellow powder in the mortar and pestle is minus the ferric nitrate).
At first, heating with a lighter causes the mix to gel and darken quickly, presumably vulcanising in situ. The blackened material can be lit
when concentrating a flame on one spot, first fizzing a bit, then takes off and makes a steady flame and smoke. The burn was very nice, controlled and
steady, it's also quieter than candy burns (doesn't have that roar). Beautiful bright yellow from the sodium.
Early success, yay!
PS: do you like my 'witness plate' 
PPS: burn and residue, it smells like old-fashioned rubber elastic bands, not very foul but rather sharp and distinctive.
   
Pictures:
[1] A tiny chunk of Mg[lin]2. This amber-like material is easy to break with a spoon, very brittle and glassy.
[2] Mortar and pestle grinding with added sulfur and NaNO3, no ferric nitrate nonahydrate yet (that imparted a reddish-brown tinge to the grind).
[3] Enflammer!
[4] Residue, note the small amount of carbon and red tinted blobs of molten Na2S residue. All-in-all, not a bad guestimated formulation, a little more
NaNO3 perhaps.
 via Imgflip GIF Maker via Imgflip GIF Maker
Just made an animation of some of the photos.
[Edited on 28-3-2015 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | What I need now is a plasticiser for these brittle glassy linseed soaps, one option is using free fatty acids of linseed oil in my formulation. My
strategy will be to use a salting out/ion exchange of the Na[lin] with NaHSO4, which is obtainable cheaply as pool dry acid.
|
That won't yield the Na soap but the free fatty acid, as it is insoluble.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by deltaH  | What I need now is a plasticiser for these brittle glassy linseed soaps, one option is using free fatty acids of linseed oil in my formulation. My
strategy will be to use a salting out/ion exchange of the Na[lin] with NaHSO4, which is obtainable cheaply as pool dry acid.
|
That won't yield the Na soap but the free fatty acid, as it is insoluble. |
Yes, that's what I'm trying to make with the NaHSO4, the free fatty acids:
Na[lin](aq) + NaHSO4(aq) => H[lin](l) + Na2SO4(aq)
Normally I'd simply use HCl, but I figured NaHSO4 is more practical and I'm having so much fun with these duel salting out ion exchanges, was keeping
with the theme 
[Edited on 28-3-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
BTW, the burn posted earlier was really freakin' bright (yellow), I think it could make a nice flare at night time. The animated gif was done in
daylight and even there you can see how bright the light is, it's really something.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | | BTW, the burn posted earlier was really freakin' bright (yellow), I think it could make a nice flare at night time. The animated gif was done in
daylight and even there you can see how bright the light is, it's really something. |
Well, that's lovely but isn't it thrust you're looking for? 
Might be an idea to stick some of that formulation into a cardboard cylinder, light that candle and see how fast it burns.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yeah, never had much love for physics though, having too much fun with the chemistry. I'm focusing on developing the propellant for now, I'll leave
the rocket building to the rocketeers 
As for just sticking it in a tube, that I think I will do when I'm happy with the formulation and not in my apartment 
[Edited on 28-3-2015 by deltaH]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quick update on my own feeble factice experiments :-
SCl2 dosing works quite well, although all the reagents need to be really cold to avoid it running away and burning the rubber.
Today's attempt to make SCl2 in situ by adding 30% w/w S to 50g of Oil, then bubbling Cl2 through it just made a thin
brown tar.
Adding a pinch of ZnO immediately formed a thin layer of Brown Stuff on top of the Oil/S mixture, so looks interesting.
|
|
|
APO
National Hazard
   
Posts: 627
Registered: 28-12-2012
Location: China Lake
Member Is Offline
Mood: Refluxing
|
|
Very nice work everyone! I'm curious to see how much the burn rate would increase for an already vulcanized sample. Also interested if an already
vulcanized sample would maintain it's previously mentioned brightness during deflagration. If the un-vulcanized sample ended up burning brighter and
slower, then maybe it could be a flare comp, and then the vulcanized sample could be a propellant comp.
DeltaH, do you still plan on revisiting the ion exchange using ferrous sulfate instead? Propellant made from Fe[lin] sounds like it would be very
good, as it would probably have a quicker burn rate due to the amount and intimacy of which the catalyst would be mixed.
Assuming any zinc oxide or stearic acid additives leads to more complete vulcanization, and that more complete vulcanization leads to higher burn
rate, then maybe the inherently higher burn rate of the Fe[lin] based propellant could give it an edge compared to other [lin]s. Meaning that maybe
Fe[lin] based propellant made with no additives, could be as good or better than other [lin] based propellants made with additives. Assuming all that
were true, then Fe[lin] based propellant could be even more amateur friendly, as the additives could be considered more optional.
Using ferric nitrate instead of ferrous sulfate and boiling down to have premixed the sodium nitrate all in one step sounds like it could be even more
amateur friendly, if it could be done in a more feasible manner as to not defeat the initial convenience. If done in a certain way, it could even end
up in the oxidizer being more efficiently mixed, leading to a better overall propellant.
Also, I thought about use of mixed nitrates to form a eutectic mix with a lower melting point, to aid in mixing of the oxidizer, but I think 220C is
still too high to be of any use.
Anyway, I look forward to your guy's progress.
"Damn it George! I told you not to drop me!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks APO. Yes, more on the ferrous sulfate will follow soon. My work needs to take a pause now for a little while, but it's far
from over. The use of ferric nitrates in an ion exchange made a hell of a mess, so I really wouldn't recommend that, but simply mixing the crystals
into the final composition with oxidant worked well i.t.o not making a gloopy mess 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Somebody suggested that i made S2Cl2 last time rather than SCl2 so i made some more today using 10x the quantity of
Sulphur and a different method.
The product is similar in appearance, yet is more orange rather than a definite pink.
Reacting with Sunflower oil, it is radically different.
The First pink liquid sizzled and scorched the oil as well as cross-linking it into a solid mass.
This Orange liquid has a much gentler heating and cross-linking behavior, also producing a solid.
Linseed oil (tin says it has drying agents) reacts faster with today's Sulphur/Chlorine product than Sunflower oil and more completely forms a rubbery
solid.
This reaction is more exothermic than with Sunflower oil, yet never got too hot to touch (small scale test).
Now to go out on a limb to see if my thinking is correct ...
The First product reacted faster, and the reaction released more heat.
That is due to greater changes in Oxidation state of the involved species (Sulphur)
Therefore the First product would be SCl2 (S<sup>+2</sup> and Todays' product S2Cl2 (S<sup>+1</sup>
and Todays' product S2Cl2 (S<sup>+1</sup> . .
Today's product appears more promising in converting oils into factice.
[Edited on 26-4-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Great! Any pics of the actual factice?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Factice Experiments #2
These experiments were done to determine the quantity of S2Cl2 required to convert liquid Linseed & Vegetable oil into a
rubbery solid factice.
In addition, 4 experiments use FeCl2 and FeCl3 to see if they have any effect at all.
12 plastic cups were pre-filled with 10 to 11g of oil and chilled in an ice bath.
Varying amounts of ice-chilled S2Cl2 was added to each one, with the mixture being stirred vigorously by hand, then left to
react for 10 minutes.
After 10 minutes each sample was photographed normally and under a microscope, then the solid/rubbery portion of of each sample was extracted (where
possible) washed in detergent, rinsed in DIW, dried then weighed.
Attached is a spreadsheet of the results and some pretty pictures.
Attachment: Factice #2.xlsx (11kB)
This file has been downloaded 355 times

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
How flexible are these samples? Literature that's been linked to above mention low Shore A hardnesses in the range of 'floppy soft', gel-like
materials (for commercial factices).
This is the first time I've seen anyone having a go at preparing home made factice. Well done.
[Edited on 26-4-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sample # 3 was most rubber-like and certainly bendy, yet if bet too far it broke in half.
Sample #8 was very similar, yet very sticky on the upper side.
#4 got burnt to buggery by the heat from the reaction and basically had almost no flexibility at all.
#5 was extremely sticky, with the appearance and stickiness you find from a well chewed soft sweet.
Packaged correctly and this material could easily make SpiderMan's web work.
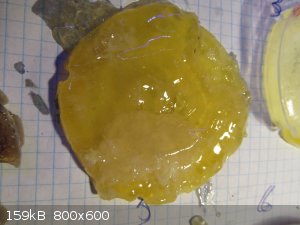
|
|
|
| Pages:
1
..
4
5
6 |