| Pages:
1
2 |
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Test for Fe2+ and Fe3+
I'm trying to put together a qualitative test kit for iron in water leached from soil samples that can detect both Fe2+ and Fe3+. My idea is to have
a bottle of dilute nitric to acidify the sample, then a solution of potassium thiocyanate to test for Fe3+. If that fails to yield the red color of a
positive test (ferric thiocyanate complex), then add sodium chlorate to oxidize any Fe2+ to Fe3+ and produce the red color.
The test seems to work well enough, but my question is, does anyone see any danger in that? Potential for formation of dangerous (explosive) chlorate
salts in the waste disposal?
I realize hydrogen peroxide would serve adequately as oxidizer, but I'd like to avoid samples foaming. I picked chlorate because of availability and
the fact that it's colorless (also because nitric failed to oxidize Fe2+ in dilute solution).
|
|
|
kecskesajt
Hazard to Others
  
Posts: 299
Registered: 7-12-2014
Location: Hungary
Member Is Offline
Mood: No Mood
|
|
I dont think so.Soil dont have so muck reducable components to make any explosive product.And in water, nitrogen triiodide is stable so keep wet the
samples.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You can use a solution of potassium hexacyanoferrate(II), a.k.a. yellow prussiate of potash for detecting the iron. This test is VERY sensitive, more
so than the thiocyanate test. It also requires very small amounts of reagents.
In practice the test works as follows:
- Add your sample to 100 ml of water.
- Add a few drops of dilute acid (e.g. 10% HCl or 10% HNO3) to your sample
- Add a drop of 3% H2O2 to 100 ml to your sample, stir and allow to settle for a minute or so
- Add a few drops of a 10% solution of yellow prussiate of potash.
If any iron is present (either as iron(II) or as iron(III)), then the liquid turns bright blue.
The reagents for this are easy to obtain. The yellow prussiate of potash stores well. No need to worry about foaming or strong acids, because the acid
reagent and hydrogen peroxide are used at great dilutions.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Thanks woelen! I knew there was a test for iron using hexacyanoferrate but I was under the impression that it only detected iron(III). If it detects
both, then I'll go with that.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Btw, woelen, why hexacianoferrate (II) detects both irons? what about hexacyanoferrate (III) ? (at least I know the latter detects Fe (II) )
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I used to do this with o-phenanthroline. An aliquot is treated with o-phenanthroline to make [Fe(phen)3]2+ (ferroin). The absorbance is measured with
a spectrophotomer vs. external standard curve (I used ferrous sulfate). This gives Fe2+.
Another aliquot is treated with hydroxylamine to reduce any Fe3+ to Fe2+, treated with o-phenanthroline, and the aborbance is measured. The total iron
is thus quantified, and Fe3+ can be given by difference.
See, for example: http://www.ebay.com/itm/1-10-Phenanthroline-monohydrate-o-Ph...
O3
[Edited on 18-6-2015 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by papaya  | | Btw, woelen, why hexacianoferrate (II) detects both irons? what about hexacyanoferrate (III) ? (at least I know the latter detects Fe (II) )
|
Hexacyanoferrate(II) detects iron(III), but the drop of H2O2 (3%) oxidizes any iron(II) to iron(III), so
this makes the test sensitive for both iron(II) and iron(III).
The hexacyanoferrate(III) (the red salt) detects iron(II), so, using a reductor, together with the red prussiate would be an option as well. The use
of the yellow salt, however, is preferrable. The red salt is light sensitive and solutions of it slowly decompose, giving many kinds of complicated
dark blue cyano-complexes (no free cyanide is produced), while solutions of the yellow salt are more stable and the solid keeps indefinitely, both in
the dark and under light.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
woelen, you mentioned that hexacyanoferrate(II) is more sensitive than the thiocyanate test, any idea what the detection limit is? ie. How low can
the iron content be before the blue color is either imperceptible or could be easily missed?
Ozone, thanks for the explanation of the o-phenanthroline test, I have a version of that from Hach. I was just looking for a quicker/simpler
qualitative version.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Having been handed the solution to your question on a plate, a little experimental work on your part would quickly give you the answer to your own
question no ?
Please do those experiments, document them, and post the results here.
I for one would be very happy to have all that careful work done for me so i would not have to expend any effort at all.
Edit:
If you are unsure of the process, discuss it here for advice, and i will spend the time to replicate the (eventual) process to confirm your results.
[Edited on 18-6-2015 by aga]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by agent_entropy  | | woelen, you mentioned that hexacyanoferrate(II) is more sensitive than the thiocyanate test, any idea what the detection limit is? ie. How low can
the iron content be before the blue color is either imperceptible or could be easily missed?[...] |
I do not know the exact figures, but one important difference between the two tests is that the thiocyanate test requires fairly high concentration of
thiocyanate. The complex with thiocyanate is labile and when the concentration of thiocyanate is low, then it tends to decompose again in its separate
ions. The hexacyanoferrate complex, on the other hand, is very stable and once it is formed, it does not decompose anymore, provided the pH is kept on
the low side of neutrality (hence the need to add a few drops of acid).
In practice, I have done this test myself and it is amazingly sensitive. If you take one little crystal (appr. 1 mm diameter) of an iron salt and
dissolve that in a liter of water and then take 100 ml of this, add a few drops of acid, a drop of hydrogen peroxide and a small spatula of
hexacyanoferrate(II), then you get a clearly visible blue color.
It should be possible to use colorimetric determination for more quantitative results, but for a simple test, which is performed on location, this of
course is not possible, when you just have a small test kit with you.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | | The complex with thiocyanate is labile and when the concentration of thiocyanate is low, then it tends to decompose again in its separate ions.
|
I don't think that is strictly speaking true.
The FeSCN<sup>2+</sup> has two disadvantages compared to Prussian Blue.
1. The formation constant (K<sub>f</sub> of
FeSCN<sup>2+</sup> is quite low, in the order of 400 to 500, IIRW. Prussian Blue is insoluble, so it has a much higher
K<sub>f</sub>. of
FeSCN<sup>2+</sup> is quite low, in the order of 400 to 500, IIRW. Prussian Blue is insoluble, so it has a much higher
K<sub>f</sub>.
2. Prussian Blue is very intensely coloured. much more intense than FeSCN<sup>2+</sup>(#).
Both points makes Prussian Blue much more sensitive. And of course it allows detection of both Fe(+2) and/or Fe(+3), assuming you have both versions
of
hexacyanoferrate.
(#)The full structure is likely to be [Fe(SCN)(H<sub>2</sub>O)<sub>5</sub>]<sup>2+</sup>, I think...
[Edited on 19-6-2015 by blogfast25]
|
|
|
Augustus
Harmless

Posts: 6
Registered: 23-6-2015
Member Is Offline
Mood: No Mood
|
|
Such a test will not work with either thiocyanate or hexacyanoferrate(II). Since water was in contact with soil and it's pH was 5 or higher then
almost all iron was absorbed by soil as Fe2O3*nH2O (don't even hope to find iron(II) in there). Concentration of iron in this water will never be
enough to be detected by thiocyanate or hexacyanoferrate(II). The only solution is to leech iron from soil samples with acid.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Are you speaking from experience ?
If so, please expand on how/what you did in your soil Fe measurements/xperiments.
If not, erm, what ?
|
|
|
Augustus
Harmless

Posts: 6
Registered: 23-6-2015
Member Is Offline
Mood: No Mood
|
|
Yep, from experience.
Second solution is to use solvent extraction (100 ml water and 1 ml CHCl3) and a reagent of choice for iron.
|
|
|
Augustus
Harmless

Posts: 6
Registered: 23-6-2015
Member Is Offline
Mood: No Mood
|
|
I used to detect heavy metals with ppb dilution in drinking water with dithizone and CHCl3 extraction.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If you could elaborate, and explain how you did those procedures, i'm sure that would be of great help to the OP, and of interest to all of us.
[Edited on 23-6-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Augustus  | | Such a test will not work with either thiocyanate or hexacyanoferrate(II). Since water was in contact with soil and it's pH was 5 or higher then
almost all iron was absorbed by soil as Fe2O3*nH2O (don't even hope to find iron(II) in there). Concentration of iron in this water will never be
enough to be detected by thiocyanate or hexacyanoferrate(II). The only solution is to leech iron from soil samples with acid. |
He would acidify the soil sample. That should be enough to leach out Fe(+3).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by agent_entropy  | | a qualitative test kit for iron in water leached from soil samples that can detect both Fe2+ and Fe3+. My idea is to have a bottle of dilute nitric
to acidify the sample |
I guess the OP has that aspect covered.
It would still be great to hear how ppb detection works.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not putting my hand in the plasma on that but ICP atomic emission spectroscopy might do it.
Having said that it would be interesting to see how low Fe(3+)/K4Fe(CN)6 goes. Not hard to do. Might be able to find it in a nook or cranny of the
Tinkernettings...
[Edited on 23-6-2015 by blogfast25]
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I'm actually hoping to NOT find free iron in the soil leachate (cleaning up an iron (III) chloride spill) so if it is indeed absorbed as Fe2O3*nH2O
that would be wonderful. However, I still need to do the tests in order to quell the concerns of others.
@aga, my potassium hexacyanoferrate (II) finally arrived today and I will begin experimenting both visually and spectrophotometrically to try to find
a detection limit.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cool.
photos would be nice along with your findings.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I just realized that spectrophotometric investigation will be essentially meaningless since Prussian blue is insoluble. I guess I'll continue
visually.

[Edited on 24-6-2015 by agent_entropy]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by agent_entropy  | I just realized that spectrophotometric investigation will be essentially meaningless since Prussian blue is insoluble. I guess I'll continue
visually.
[Edited on 24-6-2015 by agent_entropy] |
At low concentration it is colloidal. It will still colour a solution, even at very low concentrations. There are colorimetric determination protocols
based on Prussian Blue, if I'm not mistaken.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Indeed, Prussian Blue seems to be soluble or at least colloidal at low concentration.
Dropper bottles of testing reagents were prepared. 3M Nitric acid, 3% Hydrogen peroxide, 0.2M potassium hexacyanoferrate (II) (10% as the
trihydrate), and 0.2M KSCN.

A 500mL stock solution containing 10ppm iron as Fe3+ was prepared volumetrically by dissolving 0.113g Fe(NO3)3*9H2O. The stock solution was diluted
to 3, 2, 1, 0.5, 0.25, and 0.1 ppm standards.

5mL of each standard was pipetted into a small vial.

2 drops of 3M Nitric acid were added to each vial.

2 drops of 3% Hydrogen peroxide were added to each vial.

1 drop of 0.2M potassium hexacyanoferrate (II) was added to each vial.

About 10 minutes later the 10ppm sample had a precipitate of Prussian Blue.

The same procedure was repeated except that 1 drop of 0.2M potassium thiocyanate was added to each vial.

Visually, I would say that the hexacyanoferrate test becomes unreliable at less than 1ppm and the thiocyanate test becomes unreliable at less than
10ppm.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
UV-Visible spectra were recorded for both tests.
Hexacyanoferrate Test:
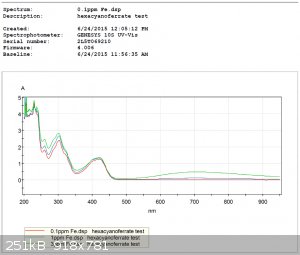
Thiocyanate Test:
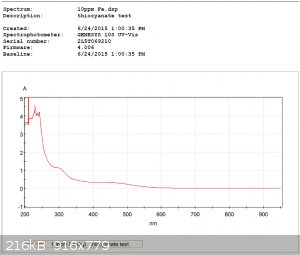
It was also possible to construct a calibration curve for each test reagent.
Hexacyanoferrate Curve (using the 700nm absorbance):
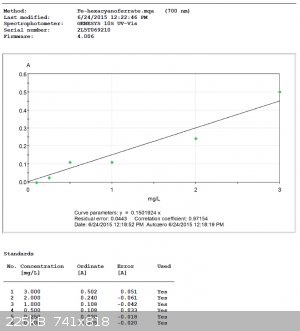
Thiocyanate Curve (using the 430nm absorbance):
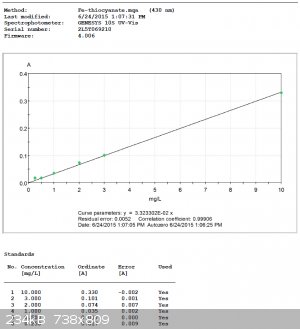
It would seem that, obviously, the spectrometer can determine colors better than the eye.
Also, no iron was detectable in any of the soil sample leachates. 
[Edited on 24-6-2015 by agent_entropy]
|
|
|
| Pages:
1
2 |