Nectarine
Harmless

Posts: 44
Registered: 19-4-2014
Member Is Offline
Mood: No Mood
|
|
Chlorosulfonylation prediction on Aromatic rings...
Hi males and females
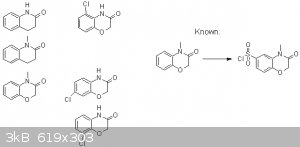
I've done some chlorosulfonylation of these aromatic quinolinone-like rings, but I don't know how to predict where the sulfonylation will happen, and
I'm not good enough at NMR to understand what peaks are going to associate with which positions of the rings...
Can anyone explain some type of prediction on which position chlorosulfonylation will occur in the following?
I've included a reaction that is known in the literature.
|
|
|
Nectarine
Harmless

Posts: 44
Registered: 19-4-2014
Member Is Offline
Mood: No Mood
|
|
Actually I believe the first substrate is known to chlorosulfonylate at the para-position to the nitrogen.
So there is some selectivity differences
|
|
|
Nicodem
|
Thread Moved
27-6-2015 at 05:11 |
Nectarine
Harmless

Posts: 44
Registered: 19-4-2014
Member Is Offline
Mood: No Mood
|
|
Up.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Well, if it is safe guessing - and nobody did it so far, then here come my theories:
Always look for the para position from nitrogen first, because it is a preferred position on aromatic amines AND sterically least hindered. ortho
position would be nice too, but the chlorosulfonyl group is a big group and I think it has difficulties to attack this position, although I would not
exclude the possibility.
If there is methyl on the N, then ortho substitution becomes even less likely to occur.
If there is O on the ring as well, then the para position to the O becomes a preffered place too. Usually N has stronger effect than O, so I'd expect
more N-para than O-para substituted products.
Cl on the ring makes para to the Cl also preferred (actually least deactivated), though reaction speed would suffer. ortho to the Cl is sterically not
a good place.
[Edited on 30-6-2015 by Pumukli]
But hey, looking at the "known" reaction my theory is broken! :-)
[Edited on 30-6-2015 by Pumukli]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
No wonder i struggle to even get started with OC.
"Big bit goes on the front end with the sticky-out bit to the top"
... might have been vaguely understandable.
[Edited on 30-6-2015 by aga]
|
|
|