| Pages:
1
2
3 |
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
if i get a cheap one and it does not work, then it is only a few $ wasted i guess. it might work as you say.
Beginning construction of periodic table display
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Do you need to detect a tiny trace of thorium in rare-earth minerals or is it sufficient to distinguish between pitchblende and hematite?
Regarding alpha/beta/gamma emissions: minerals contain all the daughter isotopes associated with the uranium or thorium parent. Many of those
daughters are gamma and/or beta emitters and can be detected with a geiger counter.
For every decay of an atom of the parent isotope, there is on average one decay of one atom of every daughter in the series/
I've made a simple geiger counter from a Z1A/J302ßy geiger tube and an electric fly squatter. Its not very sensitive, but convenient to quickly
distinguish between radioactive and non-radioactive rocks.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
Well the latest news is that i have found a source who custom builds geiger counters. i picked a russian tube (SBM20 pancake) that detects alpha beta
and gamma, filters and even the colour of the display. and the best bit is i got the whole thing for under USD100 inc shipping. so in a couple of
weeks i shall up a review for those interested.
thanks for all the info from everyone
Beginning construction of periodic table display
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Cool. However, SBM20 is not a pancake detector. Seems like it might be wise to make sure it is clear to both of you what you want/ordered.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
you're right. i actually have SBT11. the other was a cheaper option. and i was slack on previous post
Beginning construction of periodic table display
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I made my own counters from kits and vintage Russian pancake GM tubes from ebay. I'm lazy so I purchased counter kits from RHelectronics http://www.rhelectronics.net/store/diy-geiger-counter-kit.ht... particularly the one named
"ARDUINO IDE GEIGER COUNTER DIY KIT VER.2 WITH LCD; W/O GM TUBE"
They work well with the Russian pancake counters, though the dose calculator needs to be adjusted for each tube if you want some semblance of
accuracy. You need to change a variable in the software then recompile in the Arduino IDE and reupload to the counter.
The particular tubes I use with those are:
Si-22G large tube, tubular not pancake, quite sensitive but not for alpha, only beta/bgamma
SBT11A - small pancake tube, very portable, decent sensitivity
SBT10A - large pancake tube, about 4x the size of the above, very sensitive but a bit cumbersome
Si-8B - large circular pancake tube, even more sensitive than the SBT10A in my experience.
The last 3 can detect alpha.
I mounted the above tubes+counter combinations on sticks of wood, so that I can test stuff at a distance. About a 1m long stick, 5cm wide and 1cm
thick was used for this. The detector is mounted at one end of the stick, high voltage wires (rated for 1000V) connect it to the detector which is
mounted about 10cm from the other end together with a battery holder. I call them my GeigerSticks.  I had some pictures somewhere but can't find them now. I had some pictures somewhere but can't find them now.
More information on tubes https://sites.google.com/site/diygeigercounter/gm-tubes-supp...
(edit) note that you can only detect alpha radiation up to distances about 3-4cm away from the source, no matter how sensitive the tube is. That's
because the free path of alpha particles in the air is no longer than 5cm.
[Edited on 16-6-2018 by stamasd]
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
I check again prices and surprise! from 250 € some years ago now you can get one like this for...
https://www.ebay.de/itm/GM-Tube-Dosimeter-Geiger-Counter-Nuc...
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
I have a counter I can sell you for a hundred bucks. Its an old survey meter from the cold war. U2U me
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Spock
Harmless

Posts: 22
Registered: 27-3-2014
Member Is Offline
Mood: Slightly Radioactive
|
|
Out of curiosity, what type of meter is it?
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Geiger counter advice please
I want to buy a Geiger counter and I am looking at one listed on eBay with the following link:
https://www.ebay.com.au/itm/Geiger-Counter-Beta-Gamma-X-ray-...
Picture of the listing below. I have no experience with Geiger counters. The reason I want one is to check the radioactivity of my uranyl acetate
before I use it and then to check the glassware and so on afterwards. Maybe overkill but just to be sure, and something else to learn about.
If there are members on here knowledgeable about Geiger counter use, can you please comment and tell me if this one will be suitable, or if you
perhaps can suggest another model from experience?
Thanks in advance.

|
|
|
B(a)P
International Hazard
    
Posts: 1116
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Uranyl acetate is a much bigger concern from an ingestion/inhalation toxicity exposure scenario. It is also mostly an alpha emitter I think? Does that
counter pick up alpha? All in all though I don't think radiation will be you issue in handling this substance. I have not had experience handling the
substance myself though.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
From what I read so far yes it seems the radiation is so low that it is not a concern, but it is indeed toxic inside the body. Thanks for the heads up
on the alpha detection. From the eBay data listed alpha is not mentioned, but it does say it can be used to measure uranium.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I believe that a small uv lamp (or maybe LED) would cause uranyl salts to fluoresce in the visible/detectable/measurable spectral region.
It could be quite sensitive ? ... in the dark
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Online
Mood: desperate for shade
|
|
To detect alphas, you need a detector with a thin (mica?) window. Alphas have such low penetration that they will be blocked by anything more
substantial. With such a mica window, alphas are picked up easily by a geiger counter.
You will get some gammas from your U, so you should pick up something without the alphas though.
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The "specific activity" of uranium is about 25 Bq/mg
So, if very careless washing leaves a whole miligram of uranium on the glassware it will emit about 25 particles per second.
Almost all of that activity will be alpha emission- and the geiger won't "see" it because practically none will get through the plastic case.
Geiger tubes are roughly 1% efficient for gamma detection- generally, the gammas just fly through the detector without "hitting" anything.
So the total best guess is that your mg of uranium would produce about 1 click every 4 seconds.
But at best, only half of them could possibly be expected to go in the "right" direction.
So that's a click every 8 seconds- if the geiger tube is big enough to cover half the glassware.
Realistically, you might hope to get about 1 click per minute due to very dirty glassware.
Even a really good GEiger isn't going to do much better.
Maybe, if you had a good scintillation detector... What's your budget?
Fluorescence with a uv torch or uv LED is a better bet.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
How much depleted uranium acetate would one need to invest to die? How many milligrammes/grams/Oz would it take? Would you die from heavy metal
toxicity or from radiation?
|
|
|
B(a)P
International Hazard
    
Posts: 1116
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
It is not quite as simple as that, as we are all different. It is generally accepted for inhalation that less than 0.05 mg/m3 in air is safe for
adults. The immediately dangerous to life and health level is 10 mg/m3. Anything north of that is potentially going to be lethal.
For ingestion LD 50 for rats in 207 mg/kg.
The compound acts on your kidneys and over exposure usually results in death from renal failure.
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by draculic acid69  | | How much depleted uranium acetate would one need to invest to die? How many milligrammes/grams/Oz would it take? Would you die from heavy metal
toxicity or from radiation? |
It would almost certainly be the toxicity (rather than the radioactivity) that killed you.
It is difficult to see how this would matter.
This suggests that some bloke survived a gram of the corresponding nitrate
https://www.ncbi.nlm.nih.gov/books/NBK158798/bin/LSE2.pdf
But I'd not like to bet on it.
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
I have some uranyl acetate. Just be sure to not get it into your system (inhalation, digestion) since its quite soluable in body fluids. The alpha
decay would then stay in your body and attack the cells continuously and directly.
It's a weak alpha emitter, so no concerns on its radiation in the environment.
When I use my Geiger/Müller counter on the bottle it goes to up to 180 microSV.
That's basically harmless.
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
And concerning Geigercounters, they only detect gamma. But also a weak alpha emitter like uranyl acetate emitts some gamma, which will be detected by
every Geigercounter.
I think it's like 90% alpha, 6% beta and 4% gamma or something like that.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
A gamma/beta detector isn't ideal for use with uranium salts. Uranium salts contain (almost) only uranium isotopes (238, 235, 234), which are alpha
emitters, with little beta and gamma from the small quantities of decay products that have accumulated since manufacture.
Uranium ore on the other hand also contains all the decay products of uranium in secular equilibrium, each of which has an activity equal to that of
the uranium isotope it came from. At secular equilibrium, the isotopes are produced as fast as they decay, so their amount doesn't change much over
time, and they each contribute equal amounts to the radioactivity (in number of particles emitted per second).
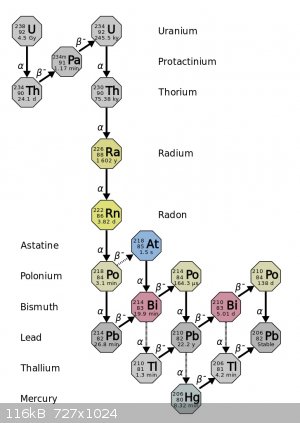
So uranium ore is much more radioactive than pure uranium and we can estimate by how much from the decay chain below.
There are a total of 8 alpha decays and 6 beta decays per uranium 238 atom to reach stable Lead-206. Uranium-238 makes up most of natural uranium and
has, by itself, an activity of about 12.5 kBq/mg. That's 12500 decomposition events per second per gram not only for U-238 but also for each decay
product, for a combined total of 8x12.5 = 100 kBq/g as alpha particles and 6x12.5 = 75 kBq as beta particles. Almost as much beta as alpha activity!
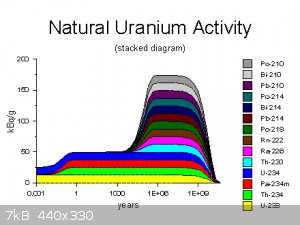
There's a nice website that explains the principle of secular equilibrium, from which I took the activity plot below. You can see that soon after
purification uranium has mostly U-238 and U-234 alpha emission. After years, you start to get beta decays from Th-234 and Pa-234m. And after millions
of years you are at equilibrium, hence why this kind of thing only happens in uranium minerals!
(The contribution of the U-235 decay chain is negligible in comparison to U-238, as U-235 has an activity of only about 5% that of U-238 in natural
ores)
Additionally, beta particles (which are simply electrons with high energies) can interact with the nuclei of atoms (especially heavy ones such as
uranium) to produce x-rays. A phenomenon known as Bremsstrahlung which is used in x-ray tubes for that exact purpose. X-rays are high energy photons,
same as gamma rays. The yield of x-rays is low but not zero. So uranium ore produces both beta particles and x-rays in addition to alphas, which is
why it can be easily be detected by beta- and gamma-only geiger counters.
If you want to use a geiger counter for safety reasons when using uranium, you should really get one which is sensitive to alpha particles. Especially
since uranium chemistry involves purification steps, such that you cannot count on the decay products to make the uranium "visible" to a beta/gamma
detector.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks members for all the info and advice.
|
|
|
Texium
|
Threads Merged
3-4-2022 at 17:10 |
Texium
|
Threads Merged
3-4-2022 at 17:12 |
Texium
|
Thread Split
3-4-2022 at 17:13 |
Texium
|
Threads Merged
3-4-2022 at 17:14 |
Texium
|
Thread Topped
4-4-2022 at 15:10 |
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Last year I finally got around to buying a device. I went with an old CD V-700 from eBay. It previously belonged to the state of Nebraska and had been
serviced/inspected a couple years before, so I figured that there was a good chance of it actually working, and it would appear that it does. It was a
good deal, and it came in the original box with the original manual, a couple dosimeter pens, and a charger for the pens. Those probably don't work,
but they're cool antiques nonetheless. The whole lot was about $200. Here's some pictures. I was able to get a faint response from a banana with the
beta shield open, and a stronger response from an americium source out of a smoke detector. Wanted to post these because I wasn't able to find much
info on what these models are capable of detecting without modification.
 
 
Given that it responds well to the weak gamma from the americium source, is it safe to say that this meter could work well for identifying hot rocks
out in the desert? I don't currently own any ore to test it with.
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I have a GMC-500 and its a really nice unit with wifi, USB and logging features.
But it does only detect Beta, Gamma and X-Ray, no alpha.
One can install different tubes in it, maybe a alpha tube is avaliable.
Its also american made, good quality.
GMC-500
|
|
|
Deathunter88
National Hazard
   
Posts: 508
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Texium  | Last year I finally got around to buying a device. I went with an old CD V-700 from eBay. It previously belonged to the state of Nebraska and had been
serviced/inspected a couple years before, so I figured that there was a good chance of it actually working, and it would appear that it does. It was a
good deal, and it came in the original box with the original manual, a couple dosimeter pens, and a charger for the pens. Those probably don't work,
but they're cool antiques nonetheless. The whole lot was about $200. Here's some pictures. I was able to get a faint response from a banana with the
beta shield open, and a stronger response from an americium source out of a smoke detector. Wanted to post these because I wasn't able to find much
info on what these models are capable of detecting without modification.
Given that it responds well to the weak gamma from the americium source, is it safe to say that this meter could work well for identifying hot rocks
out in the desert? I don't currently own any ore to test it with. |
Go look up antiprotons on Youtube, he has many in depth videos on different types of geiger counters, including your model.
What geiger counter to buy: https://www.youtube.com/watch?v=OBpi7rUh26k
Victoreen CD V-700 Model 6A
https://www.youtube.com/watch?v=MQLbUaE6CB0
|
|
|
| Pages:
1
2
3 |