| Pages:
1
2
3 |
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Could be useful for loading charges, but probably not for the manufacture itself. Kneading it is bound to reintroduce air, so wouldn't any increase in
density is lost unless you handle the product very deliberately with a minimum of reshaping?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
The data was calculated with a rather simple (only 1000 lines of code...) program of mine. It guesses the reaction, calculates temperature and finally
other detonation parameters using a few empirical equations. It has surprising accuracy, almost approaching that of expensive programs like EXPLO5. A
complete JCZ3 implementation is on its way though...
Anyway, at 40% wax (OB = -146%) the VoD is calculated to 5450 m/s and P to 132 kbar at 1.28 g/cm3. More than that and the temperature
starts to drop too low for my model.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
dornier you havent posted a video in a while 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Bert  | | Having seen vacuum degassing used to eliminate or at least minimize bubbles and voids when casting composite rocket fuel grains, I wonder if anyone
has tried adapting vacuum processing to plastic explosive manufacture? |
This would be indeed an effective way to reduce the air incorporation...but the all kneeding system must be under vaccuum.
Since following the perfect gas law: p*V=n*R*T
--> then reducing the pressure will proportionnally reduce the quantity of air incorporated.
If 10% air is incorporated into a normal process at ambiant pressure; then into identical conditions but the pressure, reducing the pressure by 2, 5
or 10 will reduce the air incorporation to 5%, 2% or 1% respectively (vs the initial 10%).
Theorically if pressure can be set to 0 mm Hg (or close because 0 is unreachable) then the air incorporation will be negligible.
Practically of course one has to work with non/least volatile HE, plasticizer and oils...considering the fact any solid has a vapour
pressure...lowering the overal pressure will increase the apparent/relative volatility of all the ingredients: so even PETN or ETN may start to
sublime from the kneeder compartiment to other unsuitable parts of the system and reduce the % of ingredients of the kneeded mass based on their
respective vapour pressure.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Dornier 335A  | The data was calculated with a rather simple (only 1000 lines of code...) program of mine. It guesses the reaction, calculates temperature and finally
other detonation parameters using a few empirical equations. It has surprising accuracy, almost approaching that of expensive programs like EXPLO5. A
complete JCZ3 implementation is on its way though...
Anyway, at 40% wax (OB = -146%) the VoD is calculated to 5450 m/s and P to 132 kbar at 1.28 g/cm3. More than that and the temperature
starts to drop too low for my model. |
Nice program and project. Thank you for the calculation at 40% wax.
Sadly I don't have such programming abilities (although I understand the concept behind code lines and iterative calculations), so I work more on
feeling, raw datas comparison and extrapolation from graphics.
But as I can see, I'm not too bad since it took me less than 1 minute to guestimate/extrapolate the VOD and pressure at 40% wax  
I wish that one day you will do like Engager and let the forum benefit from your ingenious calculation program.
[Edited on 11-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Some nagging thoughts I have had for a while.
I don't know chemistry well enough to answer this, but is there any other plastizer options that can get the job done with only 1-3% inerts?
Or another option that I have confidence in, increase the grain size so less inerts are needed, like this...
https://www.walmart.com/ip/Educational-Insights-Play-Foam-Co...
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I have played with this product (children's toy) and the air gaps can be compressed quite well.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Everything is a matter of scale.
You may enter the field of nanomaterials if the film of plasticizer becomes too thin, it will loose part of its macroscopic properties (stretching
ability, elastic ability or sticking ability) because to keep those you need to keep several molecular radius of like molecules.
You are right that increasing the particle size will reduce the surface of particle to coat with the plasticizer (active surface will be less) and
thus the quantity of it...
but it may:
1°) become less kneedable (increased viscosity)
2°) become more breakable.
3°) become more sensitive because the weakest link remains the large crystals of HE (ETN, RDX and HMX for example)
--> To test.
Sole alternative would be to foccus on active binders/plasticizers...that are dense and contribute to the explosive properties.
--> Nitrocellulose and non volatile explosive gellifiers for example.
--> Other known technical compounds, glycidyl azide/ nitrate polymers, ...
--> Other unknown polymeric compounds (I have a lot of ideas because polymers usually induce higher densities and better stability (heat and shock)
than monomers)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I don't think its possible to get a moldable plastic explosive with <3% inerts. The lowest I have seen is C4 with 9% inerts.
All the new explosives testing seems to be focusing on PBX's which are bonded with a plastic polymer and have as little as 5% inerts.
Large grain size would be hard to obtain and they would probably get broken down in size a lot while incorporating the binder with rolling and
kneading.
It looks like that playfoam is small rubber balls coated in some sort of binder that makes them stick to each other so I am guessing air gaps would be
a problem.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Philou,
To avoid the sensitivity problem I was going to form large grains from small crystals with smokeless powder and acetone. TACP if a perfect candidate
because of the small crystals, low sensitivity and positive OB! The children's product is quite mold able, maybe I can dissolve that in acetone...
but the foam might dissolve also...
Laboratory of Liptakov... this could be your claim to fame! I want to experiment with this but I can not find any PIB tape in the US...
And Dornier, I am legitimately jealous of you, remember me when you go places ...and when you are a chief engineer....
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | Philou,
To avoid the sensitivity problem I was going to form large grains from small crystals with smokeless powder and acetone. TACP if a perfect candidate
because of the small crystals, low sensitivity and positive OB! The children's product is quite mold able, maybe I can dissolve that in acetone...
but the foam might dissolve also...
Laboratory of Liptakov... this could be your claim to fame! I want to experiment with this but I can not find any PIB tape in the US...
|
Beware that the TACuP may be storage inadvisable into contact with smokeless powder and/or aceton.
TACuP is a complex of NH3 and as such the NH3 is held by the Cu(ClO4)2 but not at 100%...
so the NH3 may react:
1°) with the aceton to make aceton-imin and dark polymerisation tars (only if exposed for long)
2°) most of all, with the nitric esters to hydrolyse or amonolyse it...
The children product you referenced is said to be non-sticky. (At first I thought it was magnetic core, with rubber arround)
--> So i guess the coating is stiking to itself but not to other stuffs.
--> I wonder what it is
No PIB tape...
I'm sure bird repellent/rat glue should work just fine but you have to find the right process and order of addition of the ingredients...
--> When working at Procter&Gamble on silicon additive to allow for wrinkle resistance of clothes and easy post wash ironing...
We did make an aqueous 50% silicon solution from 80% silicon into ethanol (about 5000 cps)...allowing the 80% to fall into water resulted into
immediate precipitation and hard (very viscous) plastic chewing-gum totally unworkable (>100000 cps); reversely when allowing the water to fall
drop by drop into the 80% silicon ethanolic solution, despite a transitory moderate increase of viscosity (not unworkable at all 15000), we ended up
with a very thin liquid only 10 centipoise (thus about 10 times as viscous as water) composed of sub-micron-sized silicon beads into a water-ethanol
mix.
[Edited on 11-1-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
No PIB tape #2
This is a question I've been asking myself everytime someone said that they cant find PIB:
Why dont you guys try to get it from somewhere else ?
Gloves first come to mind. You all have rubber gloves right?
And for those living in the land of the chewing gum and Ford you probably can find some chewing gum at your local convenience store.
Or go tear some tires to get at the bladder that holds the air.
Dont cut your gasmask to pieces though 
It probably is as easy to get PIB from those sources as from bird/rat traps.
Am I failing to see something?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Herr Haber  | No PIB tape #2
This is a question I've been asking myself everytime someone said that they cant find PIB:
Why dont you guys try to get it from somewhere else ?
Gloves first come to mind. You all have rubber gloves right?
And for those living in the land of the chewing gum and Ford you probably can find some chewing gum at your local convenience store.
Or go tear some tires to get at the bladder that holds the air.
Dont cut your gasmask to pieces though 
It probably is as easy to get PIB from those sources as from bird/rat traps.
Am I failing to see something? |
Bird/Rat glue I have bought is only PIB (90-80%) and solvent like hexane (10-20% by weight) nothing else...for 2,5-3,5€/100 g into brico shop...you
can't hardly make it easier to get.
The product is also immediately mixable as such with mineral oil or organic solvent.
I was also thinking to chewing-gum (after use/chewing so you get rid of most sugar, aroma) or to Multi-fix by Pritt/Henkel for Poster (glue/paste
without solvent containing Polybuten but with a mineral charge).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I haven't done anything HE related in about a year probably, but,
I never accurately measured the crystal density of the solid HE used or the density of the polymer it was mixed with to form a plastic/putty type
explosive. However, using the published data for densities of both and calculating an average density based on proportions used always came out to
very, very, close to the product density found using good scales to determine mass and a decent graduated cylinder and careful volume measurement
using the water displacement method.
From what I saw, more kneading generally resulted in a density closer to what the calculated average density was. I have not used the solvent method
in a while. I settled on bird repellent (91% PB IIRC, I looked for the highest percentage I could find) and mineral oil. I use a bit of heat (a
previously heated, with hot water to ca. 50C, then insulated to slow heat loss, rolling board, glass cutting board for instance) and a glass rolling
pin (can be bought for baking, there are also really light (not glass) Teflon coated rolling pins that would likely work really well, or even just a
suitable glass bottle).
If I had air in most of my putty explosives, when well kneaded, it was a very small amount (I think).
BTW, good to read some of what you characters are writing about and see what you are up to again.  I have actually been tied up lately applying some of my science & engineering skill to an R & D project where
we are making synthetic diesel from wood. I have actually been tied up lately applying some of my science & engineering skill to an R & D project where
we are making synthetic diesel from wood.
[Edited on 24-1-2017 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
diesel from wood
 Making synthetic diesel from wood is my dream for all life. ...... Making synthetic diesel from wood is my dream for all life. ......
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I thought the bird repellent was not that good because it is a short molecular chain as LL stated.
It is hard to beat the ease of NM and Nitrocellulose. Are there any agents that would slow down the NM evaporation? Petroleum jelly helps, but it
seems a high amount is needed, which resulted in no detonation....
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Camphor is pretty available, solid material and soluble in esters. Next is possibility use microtene bag.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
NM with NC is a bit like using MN (Methyl nitrate) with NC.
Better use EGDN or NG with ETN and NC instead.
Or the more exotic 1,2-dinitroethan ...denser, less volatile, but less available and harder to do.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Concerning inert binder/plasticizer systems, and trouble with finding PIB:
I am presently conducting a series of experiments on a silicone (poly-dimethylsiloxane) system. It consists of curable silicone rubber caulking for
construction (the kind you buy for DIY projects) and silicone oil (bought the same places for lubricating rubber seals and such, or at the pharmacy
for lubricating... different things). It produces a mouldable product with superior mechanical properties at about 85 % inerts and seems quite
forgiving of variations in the binder/plasticizer ratio (in a range around the 50/50 mark). 85 % active ingredients seems low, but bear in mind that
silicone has a higher density than PIB/diester. If you compute the volume fraction of inerts, it is actually quite close to the value obtained for C4.
At present, I have only tried it with a non-energetic filler, in order to investigate the mechanical properties and behaviour after prolonged storage
(I'm at the 2 month mark tomorrow, and so far there has been no change).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
silicone plasticizer
Plasticizer on silicone based, poly-dimethylsiloxan oil + classic transparent silicone rubber. Results after 30 minute preparation. By 60 Celsius.

........ .........LL .........LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
today I heated a sample of the inert plastique at 85 C for 3 hours to see if I could replicate your results. However, in my case, the sample did not
exhibit any signs of change in the physical properties. The kind of transparent silicone rubber I used was neutral curing (no smell of vinegar), how
about yours? Also, did you use any solvents in the preparation?
|
|
|
Uriel
Harmless

Posts: 14
Registered: 28-1-2017
Member Is Offline
Mood: No Mood
|
|
Hello guys !
(it's my first message on this forum)
I've made research in plastic ETN.
- Motor Oil + ETN 25/75 it quitte good (23 or 24% are too few and 24 at 30 are perfect... but less powerfull of course)
- 9 Motor Oil + 1 Lecithine plastizer, about 17/18% with ETN give a plastic but that flows. Under it is powder, over it's flowing... (but good
density).
- PIB/PB (rat glue and I don't know de proportions of each) 14/15% is quite good : there is my video here :
https://www.youtube.com/watch?v=s3K67Cqzm-I
- And about 11%PIB + 4% motor oil is such good and density is better !
- I've found that the PIB can be mix under water, so, I've put ETN/Water and PIB and it's quite easy to knead, it is the method of the real C4. I let
drys many days. The density is a little better. (So better detonation pressure).
I'm pretty proud of myself on this development ;-)
For the moment, my conclusion is : if you haven't PIB you can use motor oil at about 25% (and less if you can use some Lecithine but the adhesion
falls and the plastic can flow)
If you can have PIB : about 15% is a good compromise but the density is not perfect, because there are tiny air zones (but can be improved by adding
oil, like 11%PIB + 4% oil).
I'll probably make a video of standard tests in the future.
[Edited on 28-1-2017 by Uriel]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
plastic
Good attempt. Hardcore video. Your plate I estimate on 8 mm. But 30 grams a High Quality brizant plastic, should by making this:
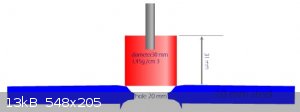
And middle quality homemade should by making hole 10 mm.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Uriel
Harmless

Posts: 14
Registered: 28-1-2017
Member Is Offline
Mood: No Mood
|
|
Hello guys !
I've made the tests !
My two plastic contains about 14% PIB by mass.
I wanted to compare ETN plastic with PETN plastic to have an idea.
As the ETN cristals are biggers, the density of ETN is quite lowest.
As ETN cristals had a density of 1.827 (1.6 is for liquid) against 1.77 for PETN, I was hoping to reach better density, but no.
I've reach a density of 1.32 for ETN and 1.36 for PETN (37mm high and 35.5/36mm for PETN)
30g in both charge with plasticizer additional.
    
As you can guess, the first one, the weakest, is ETN ans the most brisant is PETN.
Both are in the same tube of paper of exactly 30mm internal.
(And the steel plate is 10x40mm of section).
[Edited on 19-3-2017 by Uriel]
|
|
|
| Pages:
1
2
3 |