byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Preparation of 3-methoxybenzoic acid
After searching for some time I've realized that I'm unable to find any existing method for preparation of the topic compound. So I've spent some time
to perform an extensive search.
One way to prepare the compound is substitution on the third position of benzoic acid, e.g. on 3-bromobenzoyl bromide made by patent US4190600, then methoxylate with copper-sodium methoxide, but the ring is deactivated so I don't think it will work.
Another way is grignard on 3-chloroanisole US5910605. I've found a thread about preparation of 1-bromo-3-methoxybenzene, which cites a method for preparation of 3-haloanisole by substitution of 3-halonitrobenzene One-Step Preparation of Some 3-Substituted Anisoles and another thread about preparation of the precursor compound Preparation of 1-bromo-3-nitrobenzene via direct bromination of nitrobenzene Bromination of deactivated aromatics using potassium bromate; Halogenation of Aromatic Compounds by N-chloro-, N-bromo-, and N-iodosuccinimide; also there is mentioned an alternative route acetophenone->
3-bromoacetophenone -> 3-bromoacetanilide via Beckmann rearrangement (e.g. One-pot oximation–Beckmann rearrangement of ketones and aldehydes to amides of industrial interest), then 3-bromoacetanilide ->
3-bromophenyldiazonium -> 3-bromophenol.
As mentioned in those threads, 3-bromophenol price is higher than the 3-bromoanisole's one, probably because the former is made by demethylation of
the latter. But! 3-methoxybenzoic acid is cheaper than 3-bromoanisole, and even 3-chlorobenzoic acid is more expensive, so this means I've missed some
simple and cheap way of methoxyanisole production  Any ideas? Any ideas?
UPD: okay, so I've created the thread being few steps close from seeing the whole picture. Industrially 3-methoxybenzoic acid is made by methylation
of 3-hydroxybenzoic acid. The latter is made by substitution of 3-sulfobenzoic acid with sodium hydroxide in autoclave, US3094558 is probably the oldest one describing the process, also US4393234, and a procedure for substitution of mixture of 3-sulfo- and 4-sulfophthalic acid with NaOH in autoclaveUS4354038.
[Edited on 1-1-2016 by byko3y]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
UPD2: laboratory routes of preparation using a regular conc sulfuric acid and hydrolysis without autoclave: Recherche quantitative sur la sulfonation de l'acide benzoïque, abstract at J. Chem. Soc., Abstr., 1914,106, i1197-i1206, p. 1201-1202 - one of the old articles describing research of reaction conditions and catalysts on
isomers ratio and total yield, also determination of o-sulfo isomer by conversion into saccharin and selective extraction of o-hydroxybenzoic acid
with chloroform; Orienting Influences in the Benzene Ring. The Sulfonation of the Benzoic Acid - similar to the previous one, has detailed description of procedure
and a lot of links to the similar articles; The Sulfonation of Benzoic Acid - comparision of sulfonation at room temperature using fuming sulfuric acid againts hot sulfonation using conc.
sulfuric acid.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Hmmm if you want to make 3-methoxybenzoic acid, I would start from methyl benzoate.
Nitration of benzoate esters gives the m-nitro product exclusively. From there you could do a reduction to the amine, followed by a diazotization.
I don't know how the diazotization will go, but I have done the nitration and reduction years ago and it was pretty simple.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Yes, benzoate nitration gives almost exclusive m-substitution. But I have no idea how to easily substitute diazonium with methoxyl. Even if you make
m-halobenzoate - will you be able to methoxylate it? Direct methoxylation seems to be impossible - reaction of benzenediazonium compound with
methoxide gives reduction product (unsubstituted benzoate).
UPD: okay, methoxylation might be possible Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical
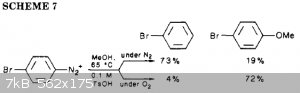
But still it's 4-5 steps ([esterification,] nitration, reduction, diazotation, methoxylation) versus 2 steps methoxylation of benzoic acid with
sulfuric acid-NaOH.
[Edited on 3-1-2016 by byko3y]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Methyl 3-chlorobenzoate should be an excellent substrate for the Ullmann reaction, since the ester is an EWG.
You could also start with acetophenone and go to 3-methoxyacetophenone followed by the haloform rxn in NaOCl.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Ullmann-type methoxylation is not Snar reaction, but a transition metal catalized coupling via oxidative insertion of metal and its reductive
elimination, so electron withdrawing group (EWG) does not simply promotethe reaction.
But you might be right that the EWG has low effect on the reaction.
The copper catalysed reaction of sodium methoxide with aryl bromides - here m-Cl aryl reacts x2.5 times faster than p-MeO.
N,N-Dimethylglycine-Promoted Ullmann-Type Coupling Reactions of Aryl Iodides with Aliphatic Alcohols - as you can see, here aromatic with electron
donating groups give higher yields (90-95%), while EWG give 75-80%. However, it's just a particular catalyst and the difference is small anyway.
So, in the end, 3-bromobenzoic acid can be easily converted into 3-methoxybenzoic acid, while the 3-bromobenzoic acid can be made using methods
mentioned in the first post.
[Edited on 3-1-2016 by byko3y]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
doesn't the EWG need to be ortho/para to the leaving group to effect the reaction ?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
CuReUS, correct statement for Snar reaction, which has nothing to deal with ullmann reaction.
BTW, any ideas on mechanism of substitution of the nitro group meta to EWG? Free radical pathway leads to 3,4,5,6 products, presence of radical
scavenger leads to 2 as a major product.
One-Step Preparation of Some 3-Substituted Anisoles
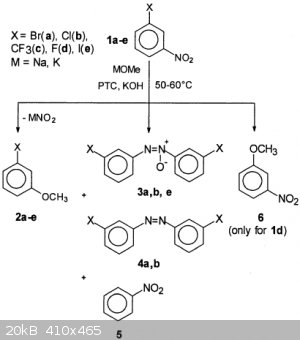
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Somewhat off topic maybe... regarding the synthesis of the possible 3-chlorobenzoic acid percursor: Could the following article be an easy, high yield
synthesis of 3-chloro benzoic acid? 
In the article they use n-chloro succimniide as the chlorinator and FeCl3 catalyst in acetonitrile to selectively produce 3-chloro methylbenzoate in
94% yield. Wondering if TCCA might also work here, despite it being a larger molecule. It would further be interesting if the methyl/ethylbenzoate is
a sufficient solvent for the TCCA and FeCl3, although the acetonitrile may actually help transfer the chlorine. I don't see a direct reason why
heating TCCA/FeCl3/methyl benzoate together could directly produce 3-chloro benzoate. After cooling ethanol might be added and cooled to precipitate
the isocyanuric acid. Any ideas?
Attachment: 1521-Tanemura.etal.Halogenation.of.Aromatic.Compounds.by.N.Cl.Br.I.Succinimide3b7d.pdf (66kB)
This file has been downloaded 444 times
[Edited on 25-4-2016 by nitro-genes]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I didn't know much about the Ullmann reaction when you said this, but I eventually learned that you're right about carbon nucleophiles (the Hurtley
reaction) but not oxygen nucleophiles (which can be significantly more reactive).
However, in this case, I suggest starting from the pharmaceutical phenylephrine, which can be oxidized to 3-hydroxybenzoic acid by potassium
permanganate, if I'm correct in assuming that an aminoalcohol will react similarly to a glycol. That way we don't need to worry about transition-metal
catalysis and all the strangeness that comes along with it.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | but I eventually learned that you're right about carbon nucleophiles (the Hurtley reaction) but not oxygen nucleophiles (which can be significantly
more reactive). |
I don't understand,could you explain it again .
| Quote: | | I suggest starting from the pharmaceutical phenylephrine, which can be oxidized to 3-hydroxybenzoic acid by potassium permanganate, if I'm correct in
assuming that an aminoalcohol will react similarly to a glycol. |
as usual,another brilliant idea  .Amazingly,I learnt about phenylephrine just
yesterday. .Amazingly,I learnt about phenylephrine just
yesterday.
I have seen amino alcohols show similar reactions to glycols(oxidative cleavage using lead tetraacetate),so it might behave similarly here as well.
what I am more worried about is using KMnO4 on a phenol ring.Unless that oxygen is covered up(by methylation),there might be formation of
quinones.But at the same time you don't want to methylate the side chain -OH or -NH.
Perhaps using a weak base like Na2CO3 will help ?
|
|
|