| Pages:
1
2 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hydrochlorination of alk-1-ene?
Does anyone have experience/information on the (hydro)chlorination of alk-1-enes with anhydrous HCl in a solvent (pet.ether, dichloromethane) by
SN1?
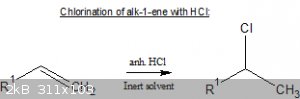
One example:
http://www.orgsyn.org/demo.aspx?prep=CV1P0166
[Edited on 10-1-2016 by blogfast25]
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Yes. The HCl adds easily to Isobutylene to produce Tert-Butyl Chloride.
Gsd
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
You may find this useful:
http://www.sciencemadness.org/talk/viewthread.php?tid=63314#...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks Magpie. What's your HCl generator there?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I heated a mixture of 95% NaHSO4 and NaCl. The mix was made up of 249.1g of oven dried Spa Down and 181.4g of food grade NaCl. This very simple
method for the generation of HCl was suggested by garage chemist.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | I heated a mixture of 95% NaHSO4 and NaCl. The mix was made up of 249.1g of oven dried Spa Down and 181.4g of food grade NaCl. This very simple
method for the generation of HCl was suggested by garage chemist. |
I like it. Better than messing with conc. H2SO4 and NaCl. Will bear that in mind, many thanks.
[Edited on 11-1-2016 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The Organic Syntheses example is a Michael addition (1,4-nucleophilic conjugate addition). It is not what you probably had in mind assuming
by the scheme you posted (electrophilic addition). Electrophilic additions of HCl only work well on branched electron rich alkenes that give
tert-alkyl chlorides as products (like the example gsd gave). Additions that give sec-alkyl chlorides (like the one on the scheme)
are less trivial.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | The Organic Syntheses example is a Michael addition (1,4-nucleophilic conjugate addition). It is not what you probably had in mind assuming
by the scheme you posted (electrophilic addition). Electrophilic additions of HCl only work well on branched electron rich alkenes that give
tert-alkyl chlorides as products (like the example gsd gave). Additions that give sec-alkyl chlorides (like the one on the scheme)
are less trivial. |
Actually, the molecule I'm considering hydrochlorinating is limonene, so that would qualify:
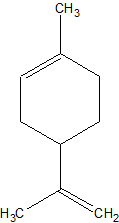
I found a ref. to it in a review paper on limonene chemistry you uploaded. But I can't find any details on the process. Pet. ether, CS2,
chloroform and CH2Cl2 were mentioned as solvents. Is a solvent really necessary here? And what about temperature? Thermodynamically the
reaction should be plenty exothermic (est. based on bond Enthalpies).
And how to protect that second double bond in the hexenyl ring?
[Edited on 11-1-2016 by blogfast25]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I've had such experience. I've used silica gel as a catalyst for hydrochlorination of butene with hydrochloric acid-dichloromethane solvent. Without a
catalyst the reaction rate is not noticable.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  | | I've had such experience. I've used silica gel as a catalyst for hydrochlorination of butene with hydrochloric acid-dichloromethane solvent. Without a
catalyst the reaction rate is not noticable. |
Which butene isomer? Which reaction products? What temperature?
Silica gel sure pops up a lot in the context of alkene hydrochlorinations...
[Edited on 11-1-2016 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Yes, it qualifies very well for an electrophilic addition of HCl.
The procedure is very simple, but you will need a distillation column for isolating the product (DOI: 10.1016/0040-4020(82)80261-5). It is an Indian
article and it refers back to an Indian article as the origin for this method, but it is from 1982, so it may be fine. As a bonus it also describes
SN1 many solvolysis reactions of this chloride with various nucleophilic solvents.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
Yes, it qualifies very well for an electrophilic addition of HCl.
The procedure is very simple, but you will need a distillation column for isolating the product (DOI: 10.1016/0040-4020(82)80261-5). It is an Indian
article and it refers back to an Indian article as the origin for this method, but it is from 1982, so it may be fine. As a bonus it also describes
SN1 many solvolysis reactions of this chloride with various nucleophilic solvents. |
Thank you. Distillation column no problem.
But wouldn't hydrolysis in mild conditions with weak NaOH/KOH be simple to obtain the carbinol? I know there's a danger of dehydrating back
to the alkene...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | | But wouldn't hydrolysis in mild conditions with weak NaOH/KOH be simple to obtain the carbinol? I know there's a danger of dehydrating back
to the alkene... |
It is a tertiary alkyl chloride, so SN2 substitutions are not possible.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yup, should have seen that one coming. 
This patent claims to use 'bleaching clay' (Fuller's earth) as catalyst for the hydrochlorination of isobutylene.
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Magpie  | | I heated a mixture of 95% NaHSO4 and NaCl. The mix was made up of 249.1g of oven dried Spa Down and 181.4g of food grade NaCl. This very simple
method for the generation of HCl was suggested by garage chemist. |
I like it. Better than messing with conc. H2SO4 and NaCl. Will bear that in mind, many thanks.
[Edited on 11-1-2016 by blogfast25] |
There is a much better way to generate HCl gas :
Drop a conc. solution of HCl (i.e. 32 or 37%) into conc H2SO4 with stirring. That is waaaay more efficient and easier to control. Note that the gas
can be passed through a washing flask with H2SO4 to be anhydrous.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Literature research ('Google scholar', mainly  ) on the hydrohalogenation (HCl,
specifically) of: ) on the hydrohalogenation (HCl,
specifically) of:
.... 2-substituted 1-alkenes (specifically limonene) yielded quite a few results but much frustration too. Various catalysts like copper, copper(I)
salts, AlCl3, SnCl4, BiCl3, silicagel and others have been reported with or without solvents, with usually long
reaction times up to 12 h. Back to back comparisons or meta-analysis don't appear to be available.
An interesting methodology is in attachment to this post (thanks to Mayko, again). Here thionyl chloride (SOCl2) with silicagel as proton donor is used with
near-complete chlorination of limonene in a matter of an hour of reaction time (and less) with very good yields.
This thesis does quite a good job of explaining how silicagel acts as a proton donor in these conditions.
Thionyl chloride is of course a bit of a kerfuffle for most hobbyists.
[Edited on 19-1-2016 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  |
.... 2-substituted 1-alkenes (specifically limonene) yielded quite a few results but much frustration too. Various catalysts like copper, copper(I)
salts, AlCl3, SnCl4, BiCl3, silicagel and others have been reported with or without solvents, with usually long
reaction times up to 12 h. Back to back comparisons or meta-analysis don't appear to be available. |
Is there any particular reason on why you prefer it more difficult? What is wrong with the hydrochlorination of limonene that I found for you?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
Is there any particular reason on why you prefer it more difficult? What is wrong with the hydrochlorination of limonene that I found for you?
|
Are you referring to:
| Quote: | Yes, it qualifies very well for an electrophilic addition of HCl.
The procedure is very simple, but you will need a distillation column for isolating the product (DOI: 10.1016/0040-4020(82)80261-5). It is an Indian
article and it refers back to an Indian article as the origin for this method, but it is from 1982, so it may be fine. As a bonus it also describes
SN1 many solvolysis reactions of this chloride with various nucleophilic solvents. |
That paper is very interesting but from what I can see of it it's about solvolysis of alkyl halides, not hydrochlorination of limonene.
Re. the latter, only thionyl chloride + silicagel seems to permit running the reaction in reasonable reaction times (about 1 h), with good yields. 6 -
12 hours is not an option for me.
[Edited on 21-1-2016 by blogfast25]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Did I miss something, or is the end goal to convert C=C w/ a tertiary and secondary carbon to C(-OH)-C with the chlorination simply being a middle
step? Is that what the dilute NaOH solution you talked about was for? If so, what about acid-catalysed addition of water to the double bond? I don't
know the mechanism for that reaction, so I don't know if it works for tertiary carbons.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The Volatile Chemist  | | Did I miss something, or is the end goal to convert C=C w/ a tertiary and secondary carbon to C(-OH)-C with the chlorination simply being a middle
step? Is that what the dilute NaOH solution you talked about was for? If so, what about acid-catalysed addition of water to the double bond? I don't
know the mechanism for that reaction, so I don't know if it works for tertiary carbons. |
Yes, the end goal is a t-alcohol (carbinol):
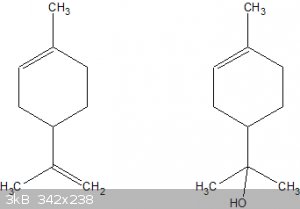
Left: limonene, right: the desired carbinol.
But a direct alkaline hydration of the 1-ene double bond doesn't appear to be possible.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's funny, E-Z Organic Chemistry by Bruce A. Hathaway talks about "acid-catalyzed hydration of double bonds" which includes hydronium
ions hydrating the double bond, which may include secondary and tertiary carbons. Though regardless of the work-ability of that route, it would
probably hydrate both double bonds.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | | That paper is very interesting but from what I can see of it it's about solvolysis of alkyl halides, not hydrochlorination of limonene.
|
Instead of claiming that it isn't there, you could have taken 5 min to read the article again. Obviously, if I said the procedure is there, it is
because it is there. Your desired product, is their model substrate after all.
| Quote: | | Re. the latter, only thionyl chloride + silicagel seems to permit running the reaction in reasonable reaction times (about 1 h), with good yields. 6 -
12 hours is not an option for me. |
You do realize that limonene is too reactive toward HCl for any such methods? You want to avoid the presence of any catalysts when your substrate is
an extremely reactive nucleophilic 1,1-dialkylethene with a possible selectivity issue. I thought you realized that already in one of your posts
upthread.
| Quote: | | But a direct alkaline hydration of the 1-ene double bond doesn't appear to be possible. |
He was talking "about acid-catalysed addition of water to the double bond", which works by the same mechanism as the addition of HCl. It was you that
posted at least two dozens of posts on this reaction in another thread.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
Instead of claiming that it isn't there, you could have taken 5 min to read the article again. Obviously, if I said the procedure is there, it is
because it is there. Your desired product, is their model substrate after all. |
I don't have full access to that paper, only its abstract:
| Quote: | | Reaction of allylic, benzylic and tertiary alkyl halides with zinc oxide in protic solvents leads to the formation of the corresponding alcohol,
ethers and esters in good yields. The scope and limitations of this reaction have been examined. The possible involvement of ion quadruplets in the
reaction is suggested. |
From there I can't glean it provides a methodology for hydrohalogenation of limonene. For that reason I didn't request the full paper. Sorry. I will
request it now.
Quote: Originally posted by Nicodem  | | You do realize that limonene is too reactive toward HCl for any such methods? You want to avoid the presence of any catalysts when your substrate is
an extremely reactive nucleophilic 1,1-dialkylethene with a possible selectivity issue. I thought you realized that already in one of your posts
upthread. |
Re. thionyl chloride, the paper I requested and Mayko provided (DOI:10.1080/00397910008087247) is explicit on the use of thionyl chloride + silica gel
(as proton donor) with limonene with excellent yields and short reactions.
[Edited on 21-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The Volatile Chemist  | | That's funny, E-Z Organic Chemistry by Bruce A. Hathaway talks about "acid-catalyzed hydration of double bonds" which includes hydronium
ions hydrating the double bond, which may include secondary and tertiary carbons. Though regardless of the work-ability of that route, it would
probably hydrate both double bonds. |
Sorry, TVC, got confused there. Long day. 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Understandable. It's like, 5:00pm there, right?
Anyways,would the method I proposed actually hydrate both double bonds? I don't exactly follow the discussion about the requirements for primary,
secondary, etc. carbons in the bond.
|
|
|
| Pages:
1
2 |