| Pages:
1
..
56
57
58
59
60 |
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Indeed it does.but it's a choice between two bad choices.pick your poison.hard to reach high temps or lower temperature, flammable hydrogen gas and
hot lead.if your at risk of H2 or lead fumes you've got bigger problems with hot p4 fumes.but then again babysitting a 1000'c+ vessel of boiling
phosphate salt with phosphine,p2o5 & carbon monoxide being evolved and trying to cool that down without breathing it in or getting it on you
doesn't sound to me like less of a risk.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The choice is doing the reaction the right or wrong way and you are choosing wrong. The wiki for the salt says the mp is over 1000C. The hydrogen
reduction is an article and a patent; the process is not shrouded in mystery. More mysterious is C instead of H.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
If it's only mention is a patent then it's not worth more than a mention.it is a nice idea with its balanced equation leaving lead water and
phosphorus but if it's not a proven pathway forget it.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The article and patent is from the govt. labs at Oak Ridge. Their address is P.O. Box X. I'm short on quartz tubes for now, and when I order big nice
glazed porcelain boats I receive tiny crude unglazed clay boats.The authors cite as inspiration an author in a French journal and year, (Hutter,
Annales de chimie 1953) who apparently tried hydrogen reduction of several phosphates, in a tube furnace, perhaps.
[Edited on 24-8-2019 by S.C. Wack]
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Phosphoric acid vapor fed through charcoal in tube furnace at 1000 C under partial vacuum while condenser traps water
I have put a bit of thought into methods to prepare white phosphorus, and the following is the safest and most accessible method that I could devise.
I have not tried it yet, since I would like to get the input of others who may have more experience. I tried posting my method on the r/chemistry
subreddit to ask for advice. After receiving ~35 upvotes I was subsequently banned. I made my case and promised to not discuss the preparation of
white phosphorus there again, and they unbanned me under the provision that I promise to "not post exceedingly dangerous experiments". 
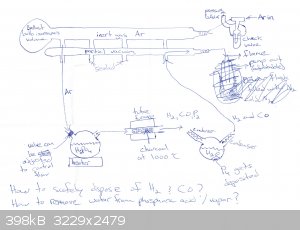
I have attached a jpeg with a schematic of my method. I advise you to take a look at it while reading this description. First the system will be
flushed with argon gas and vacuum pumped. While that is being done the glassware can be heated a bit to help any moisture evaporate. When that is done
a partial vacuum will be developed in the system for reasons that will become apparent.
The method involves packing an alumina tube with charcoal and heating it to 1000 C in a tube furnace. The choice of 1000 C is somewhat arbitrary. Some
sources have said this reaction will occur at 850 C, so I have chosen 1000 C to be safe. I will experiment to figure out how the temperature affects
the yield. I am open to other suggestions on what to use for the tube material. I have chosen alumina for its low cost and high heat resistance.
Phosphoric acid forms metaphosphoric acid when heated to 316 C. This is undesirable since metaphosphoric acid will probably not react with the carbon
in the way we want, to produce phosphorus gas. However the boiling point of phosphoric acid at atmospheric pressure is 407 C [1]. Therefore in order
to ensure that the phosphoric acid does not decompose before it can react with the carbon in the furnace, a partial vacuum is created in the system.
Then the phosphoric acid can be heated to a temperature under 300 C, vaporizing it so that it can be sucked into the furnace by vacuum pressure, while
ensuring it does not form metaphosphoric acid prior to the desired reaction:
4H3PO4 + 16C -> 6H2 + 16CO + P4
There is another problem. The phosphoric acid contains water, and it will reduced by the carbon in the furnace to produce hydrogen gas and carbon
monoxide. The danger of these gases in of themselves is not that difficult to address. These could be vented into a flame to oxidize them:
2CO + O2 -> 2CO2
2H2 + O2 -> 2H2O
My concern is that by Le Chatelier's principle if hydrogen gas and carbon monoxide build up in the tube furnace, then the equilibrium will shift so
that the reaction between phosphoric acid and carbon will be less favored. Therefore in order to maximize the yield of elemental phosphorus we should
remove as much water as possible before the acid/water vapor enters the furnace. That requires more research on my part. I would appreciate advice.
Vacuum pressure will suck gases out of the tube furnace, and passing them through a condenser will allow us to distill out the phosphorus, which will
be deposited at the bottom of a Schlenk flask underwater. The water in the flask that collects the white phosphorus may evaporate under the partial
vacuum, and that is why there is a second condenser in between the flask as the vacuum pump.
Careful research and setup needs to be done to ensure the phosphoric acid will be volatile and not heated above 300 C prior to entering the furnace,
while also not making the partial vacuum so strong that the water evaporates beyond the ability of the condensers to trap it in the flask.
[1] https://pubchem.ncbi.nlm.nih.gov/compound/Phosphoric-acid#se...
[Edited on 11-5-2020 by Duff]
[Edited on 11-5-2020 by Duff]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You need to review your understanding of the basics of chemistry if you think that diphosphoric acid will not react with carbon when orthophosphoric
acid will. You can't make phosphorus while you're still at the stage of fitting chemicals into reactions like they're ingredients for cupcakes.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
I meant to refer to metaphosphoric acid HPO3 which forms when H3PO4 is heated to ~316 C IIRC. I have sources that claim metaphosphoric acid will not
react with carbon to form phosphorus, at least not appreciably. It's not hard to believe since HPO3 has two double bonds. That is why my method
involves lowering the pressure in the system, so that the acid can be vaporized and passed over the coals at a low enough temperature to prevent the
formation of metaphosphoric acid. I'll try to find a source for the temperature where HPO3 begins to form from H3PO4, and a source for the low
reactivity with carbon.
Attachment: WO2010029570A1.pdf (592kB)
This file has been downloaded 458 times
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Hm, very interesting patent file. But if I would run this at "home" I would do this in a quarz tube an arrange the substances described from left to
right in the following way:
Beginning with a quarz wool plug (that the carbon powder will not escape, then 2 inc. carbon, then the mixture of carbon with the phosporos acid, then
again 2 inc. carbon then again the quarz wool plug that the mixture will not escape.
Through the pipe a fancy flow of nitrogen.
Then I would place over the 2 inc. carbon on the left and the right an electric heating element which can produce 900°C and start heating both
sections to that temperature. After that I would start heating the middle section maybe with a gas burner or also electric... whatever.
It the story of the patent is true, the nitrogen flow will transport the phosphorus in the colder regions of the quarz tube (which may end in some
water...
What do you think of that design?
Maybe there is an issue with Phosporus acid an quarz at that temp? But if so maybe a ceramic tube or whatever will do the job.
You cannot produce continous, but for homebrewed things I would be happy if it works also in charges. 
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Filtering out soot particles so that they do not get sucked out of the furnace is a good idea. Quartz wool might work. Argon is probably a better
choice than nitrogen for an inert gas since if there is any water vapor in the furnace it will get reduced by the carbon to form hydrogen gas and
carbon monoxide. The hydrogen gas could react with nitrogen to form ammonia, or even radicals consisting of hydrogen and nitrogen.
I don't know anything about the reaction kinetics of phosphoric acid with carbon at 850-1000 °C, or the rate of formation of metaphosphoric acid as
phosphoric acid is heated. I'm not even going to try to work out the rate laws since I think it would be a waste of time thinking about all of the
possible elementary steps and mechanisms. Let's just assume two things:
1) There is a lower bound on the pressure in the system that would make it possible to trap water in the right-hand flask with the condenser.
2) There is an upper bound on the pressure in the system that would make it possible for phosphoric acid to evaporate from the left-hand flask at a
temperature below what is required to metaphosphoric acid to form.
Then a preliminary pressure in the system must be established between those lower and upper bounds. The lower bound is determined by the effectiveness
of the condenser. A condenser with a circulated mixture of propylene glycol and water could be chilled considerably, and perhaps that would be
sufficient to make the lower bound smaller than the upper bound, so that the process is feasible.
When the system is first flushed system with argon a uniform pressure will be established in the system throughout, from the left-hand flask
containing phosphoric acid to the pump, but note that the reaction between phosphoric acid vapor and carbon is going to increase the pressure in the
furnace by producing multiple gas molecules; phosphorus, hydrogen, and carbon monoxide. Therefore if we want to maintain some range of pressure in the
furnace, gas would need to be let out of the furnace faster than it is let in. That makes the problem much easier, since the pressure on the
right-hand side of the system will be higher than on the left-hand side as the gases flow out. That is very convenient since it will make it easier to
trap water in the flask while vaporizing the acid.
A two stage regulator might be the perfect type of valve for this job. It would allow us to keep the pressure in the phosphoric acid flask low while
the pressure in the furnace and right-hand side is higher.
Anyway, I will have to experiment to find the lower and upper bounds, as well as the temperatures/pressures where metaphosphoric acid begins to form,
and all of this is based on the assumption that the story that patent is selling is even true. 
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
In my unprofessional and likely useless opinion, I suggest that you try this on a very small scale first, before spending money on exotic glassware.
If you put your trust in patents, you will oftentimes be sorely disappointed in the final results, spending too much money and time in the process.
As a curiosity I made a tiny amount of phosphorus in a borosilicate test tube once. This was done according to this equation:
6NaPO3 + 10AI + 3Si02 = 3Na2Si03 + 5Al203 + 3P2,
that was first introduced to this forum here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=65...
I simply stuck a single-hole stopper in the end of the glass tube and pulled a good vacuum using a two-stage rotary vane pump. To protect the pump,
I also used a 1-2 cubic foot vacuum reservoir. I pumped down the reservoir, and then closed off a valve to isolate the pump. The reservoir was then
used to maintain a vacuum on the reaction mixture as it was heated.
I used a propane torch to heat the reaction mixture. The borosilicate tube was barely able to handle the temperatures needed while under vacuum. It
gradually caved in as the reaction progressed, rendering it useless for future use. If the reaction started at maybe 100C lower then the tube might
have survived.
As the reaction progressed, a ring of white/yellow/red phosphorus condensed in the cooler parts of the tube. The vacuum, among other things, kept
convective heat loss to a minimum, and kept the upper parts of the tube cool.
After I noticed a sufficient amount of product (and the tube's integrity was beginning to look questionable), I stopped heating and let things cool
down a bit. Then I cracked the stopper just a bit...and a big flash of light filled the tube. Turning off the lights showed the otherworldly glow of
slowly burning phosphorus gasses swirling around the tube.
At a different time, I heated 1mL of 85% phosphoric acid in a test tube with a few small bits of metallic lead added to it. After some prolonged
heating, and with the lights turned off, a glowing ring of phosphorus/phosphine was noted at the upper end of the tube so long as I kept applying
strong heating. I did this in a fume hood, of course.
Heated phosphoric acid attacks glass. I would be careful doing that while also applying a vacuum. Protect your vacuum pump, and also protect "you"
if you end up suffering an unplanned glass implosion somewhere in the apparatus.
NaPO3 is often used (from reading the thread), because it is a relatively low melting point phosphate salt, and it contains no hydrogen. This avoids
the possibility of producing the gaseous and toxic product phosphine during the reaction.
In light of your proposed reaction, I would suggest first packing a small amount of your reactants (phosphoric acid mixed with charcoal, not graphite
powder) into an extra long test tube and heating it gradually under vacuum, just to see if there is any reaction at all under strong heating. To do
this more exotically, use electric heating around the test tube, and heat the tube inside of a vacuum chamber to equalize the pressure on the tube and
prevent it from collapsing under vacuum.
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
I fully agree. Not that I think about trying this out. I was only thinkin how I would "translate" the patent content in an apparatus which I think I
can handle better than graphite pot...
I don't need phosphor I have filled my time with nitrogen and it's oxides  , ,
But it's always interesting to learn how other people are dealing with problems and how creative they are in finding cheap and sometimes simple
solutions!
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Your opinion is greatly valued. I am not a professional either. I think I got a D in high school chemistry. I skimmed through a few chemistry books
recently to recall whatever I did manage to learn, but after skimming through 58 pages of this thread I've realized what others have mentioned. This
is really an engineering problem and not a chemistry one. Not that I am a professional engineer either.
The equipment I would have to buy for this process will be useful for other processes I am interested in, so if doesn't work out I will not have
wasted my cash. The reaction between phosphoric acid and carbon has already been reported to happen on a small scale, in this thread, and even in old
chemistry manuals. See the attached pdf, taken from the 412th page of the Manual of Chemical Technology (1897) by Rudolf von Wagner. The question is
about yield. Can we improve the yield of phosphorus by preventing the formation of metaphosphoric acid? There is no
way to know unless I perform my process as described.
The major pieces of equipment I need are:
1) Schlenk line and flasks
2) Tube furnace
3) Alumina or quartz tube
4) Vacuum pump with regulator
5) Two stage regulator valve for connection between phosphoric acid flask and furnace
6) Pressure relief valve for connection between furnace and water flask
7) Argon cylinder
I have other uses for that equipment anyway, so I might as well try.
Attachment: manualofchemicalpage412.pdf (81kB)
This file has been downloaded 422 times
[Edited on 13-5-2020 by Duff]
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Hm, the attached phosphorus paper deals with temps much higher then 850C something about white glowing so 1200C...
This experiment is shown several times on Youtube operating outside with a isolated gas oven and a metal can instead of a ceramic Retort and it woked
very well... a little bit nasty but realistic so get some 100g Phos. if one is patient.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Every YouTube video I've seen makes only a few grams out of each run.totally not worth the effort except for novelty. To make 100g would take a long
time and lots of gas and heaps of empty paint tins
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
OK, I see, so what would you suggest if 100g charge should be done in one run? Would you stick at a high temp. process based on Ca3(PO4)2, or would
you use other phosphates? e.g. sodium pyrrophoshate.
Is there an electrolytic way?
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
The attached article published by Canadian Science Publishing (formerly NRC Research Press) shows that heating has little effect on the composition of
strong phosphoric acid. However it does show that there is a relationship between concentration and composition. I trust this research more than the
patent I posted earlier, and it suggests that lower concentrations of phosphoric acid should be preferred. That goes against my initial assumption.
In fact much of what I initially assumed seems to be wrong. Oligophosphoric, polyphosphoric, and metaphosphoric acids form by the condensation of
water.
From Wikipedia:
| Quote: |
For example, pyrophosphoric, tripolyphosphoric, and tetrapolyphosphoric acids can be obtained by the reactions
2H3PO4 → H4P2O7 + H2O
H4P2O7 + H3PO4 → H5P3O10 + H2O
H5P3O10 + H3PO4 → H6P4O13 + H2O
Condensation between two –OH units of the same molecule, on the other hand, eliminates two hydrogen atoms and one oxygen atom, creating a cycle, as
in the formation of trimetaphosphoric acid:
H5P3O10 → H3P3O9 + H2O |
In the attached article 68.8% w/w phosphoric acid is found to contain ~100% orthophosphoric acid. The heat of formation for these phosphoric acid
molecules decrease (become more negative) as they join by condensation, and therefore in order to make the heat of reaction as small as possible for
the reduction of phosphoric acid by carbon a concentration of phosphoric acid not greater than 68.8% w/w should be used.
Now I see what has gone wrong in prior attempts. As the phosphoric acid is heated water evaporates, and then condensation to form larger phosphoric
acid molecules becomes energetically favorable. It is the condensation of water which occurs during heating that causes the formation of
larger phosphoric acid molecules. It is difficult to reduce these large phosphoric acid molecules using carbon since their heat of formation
is so low. Then the best method to maximize the yield of phosphorus is to use 68.8% w/w phosphoric acid while preventing the condensation of water,
but how can we prevent the condensation of water while heating the acid?
Attachment: v56-102.pdf (651kB)
This file has been downloaded 603 times
[Edited on 16-5-2020 by Duff]
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Update: I decided that the best way to avoid condensation reactions resulting in the formation of larger phosphoric acid molecules is to avoid heating
the acid until the last moment when it comes in contact with the hot carbon. A peristaltic pump can deliver a room temperature mixture of aqueous
phosphoric acid and carbon powder into the furnace.
I've ordered much of what I need to try my method. So far I've bought the following:
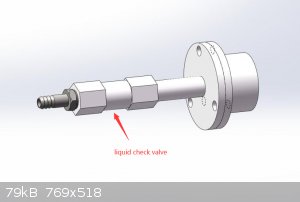
Tube furnace from China, including quartz tube, flanges, thermocouple, temperature control, ... Actually the manufacturer in China
worked with me to install a custom check valve on the furnace to allow fluid to flow in only one direction, see the attached picture they gave me.
Total cost $1500 USD
Schlenk line from ebay. Got a used Chemglass one for $410 USD
2 brand new Chemglass Friedrichs condensers for $170 USD
Won an auction for a laboratory vacuum pump on ebay for $125 USD
1 gallon phosphoric acid 85% lab grade $100 USD
39 oz activated carbon lab grade $10 USD
other knick-knacks like vacuum grease, fountain pumps, peristaltic pump, tube clamps, mineral oil $150 USD
I still need to buy an argon canister, two stage regulator (might need 2 of these), adapters for Schlenk line to hose, tubing, flasks and beakers,
bubblers, check valve for inert gas line, and other laboratory knick-knacks like safety stuff.
Yeah uh, it's all coming together. I already have a good conception in my mind of all of the little details on how the preparation will be done, I'll
write it down sometime and share all the gory details. It'll take a month or two to get all this stuff and set it up, but I'll be sure to share when
I'm done. So far I've spent $3500 CAD, and I wanted to stay under a $5000 CAD budget for this project. I probably will have to go over a budget a
little bit, but oh well.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
God I wish they would just ban pseudoephedrine instead of banning elements like iodine and phosphorus.
When I get a cold, I don't go crazy with crap from the drug store (unlike my sister who hoards a collection of like 4 OTC medicine bottles every time
she gets a respiratory infection). I tough it out.
[Edited on 12-6-2020 by Cou]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
They already have around here. It's now prescription only.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
https://youtu.be/NxQrqXIMEh8
Where can I buy the equipment in this video? The propane torch, the steel vessel heated above the tank, and the long clear condensation tube, and the
retort tube?
|
|
|
B(a)P
International Hazard
    
Posts: 1110
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
The steel vessel is just another propane tank with the tap removed and the handle/tap guard cut off.
You can buy flame weeders or heating wands for laying asphalt that you could use for the torch.
Otherwise it looks like they have just found some metal fittings that will go into the top of a propane tank and an expander to go out to the larger
steel pipe that is used.
I wouldn't know where to start with the glass receiver, maybe a giant vase would suffice, but maybe it needs to be borosilicate glass....
Edit - a quick look on alibaba shows plenty of options for the closed glass tube
[Edited on 17-6-2020 by B(a)P]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
what about this video, what is heating the mix?
https://www.youtube.com/watch?v=mibM4WUx74Q
any other details of the video? What is the mix here?
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Three yars later I stumbled upon this thread and found my failed attemps te make P4 in a 1400 C retort.
Quote: Originally posted by Magpie  |
"Magpie: How to make NaPO3 ? Or can I order"
Order sodium hexametaphosphate from Kyantec.
FYI: mp for Ca(PO4)2 is 1657°C. With (NaPO)6 mp is about 800°C IIRC.
|
I want to try it again and ordered 1kg of (NaPO3)6 via ebay.
I have a retort which should withstand at least 800 C.
Over 1200 C I don't do anymore with mild steel retorts as they burn and possibly melt.
Here a picture of two parts from plumbing pipes, the top one is a copper tube, so I want to mix (NaPO3)6 + SiO2 + C in the retort, dip the copper tube
into water and heat it to 800 C.
Would this work, or should I make a different retort ?

|
|
|
Ubya
International Hazard
    
Posts: 1231
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by metalresearcher  | Three yars later I stumbled upon this thread and found my failed attemps te make P4 in a 1400 C retort.
Quote: Originally posted by Magpie  |
"Magpie: How to make NaPO3 ? Or can I order"
Order sodium hexametaphosphate from Kyantec.
FYI: mp for Ca(PO4)2 is 1657°C. With (NaPO)6 mp is about 800°C IIRC.
|
I want to try it again and ordered 1kg of (NaPO3)6 via ebay.
I have a retort which should withstand at least 800 C.
Over 1200 C I don't do anymore with mild steel retorts as they burn and possibly melt.
Here a picture of two parts from plumbing pipes, the top one is a copper tube, so I want to mix (NaPO3)6 + SiO2 + C in the retort, dip the copper tube
into water and heat it to 800 C.
Would this work, or should I make a different retort ? |
during operation the reaction mixture should foam a bit, so remember to let enough free space
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
What are the ingredients/ratios for each mixture?
What is working best?
I will compile a list of ingredients/ratios that are working
EDIT - Nevermind, I am lazy, these pages are long, and there is 58 of them.
[Edited on 6/25/2020 by Yttrium2]
|
|
|
| Pages:
1
..
56
57
58
59
60 |