Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Corundum synthesis
Hi there
I was investigating a way to synthetize rubies/sapphires, having the availability of a muffle that can reach up to 1600°C.
I've read that ruby is usually made by mixing Al2O3 powder with 1-2% Cr2O3 and some method mentioned an
high-melting solvent, even though I wasn't able to figure out what solvent they were talking about.
Since it was mentioned that it had an high melting temperature, I tought of some metal flux; however, taking a look at some colleague's procedure, he
uses some solvent that has a density of 0,90g/cm3 and is used up to 30% wt/wt of the whole mix, in order to ease the diffusion of aluminium
and chromiun inside the structure of the future ruby.
Since I couldn't think of some metal/metal-oxide with such low density (the only metals with such low density are Na and K which would vaporize at
those T and would easily react in a N2/O2 atmosphere, besides I have my doubts whether they would be so unreactive towards alumina), I thought of some
organic under pressure, even though how the hell can it remain "unharmed" after 1600°C and be easily separated afterwards to give out pure ruby is
beyond me.
Do you have any idea of what this "solvent" might be? Also, is it possible that this synthesis takes place at temperatures inferior to the melting
point of corundum (>2000°C) and without applying pressure? And in that case, how long would it take to obtain crystal clear rubies with such
procedure?
Thanks for your attention
[Edited on 19-1-2016 by Metallus]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The solvent is going to be some ionic compound. I could guess what they might be, but they'd just be guesses.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Gemstones? See here!
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
@ DraconicAcid
Go ahead with the wild guesses, maybe we can narrow down what it is. I'll subtly ask for more info about the solvent used, so that we can rule out
some.
@hissingnoise
I've read the topic, there are some suggested DIY methods, but most give out only a little bit of red sand that has to be separated from other
impurities.
I'm aiming at obtaining some good chunk of ruby and I have the availability of good furnaces (Potentially even a machine used for Hot Pressing that
can reach up to 1900°C in vacuum).
Given these tools, is there some way to get a nice ruby platlet? If I pressed Al2O3 and Cr2O3 powders with
a static press and then put the platlet in the HP (Max conditions: 30MPa and 1900°C), would it be possible to obtain the desired product? How much
time would be required?
And given a furnace that can go up to 1200°C in air at atmospheric pressure, would it be possible with the proper solvent to obtain crystal clear
rubies?
I've tried investigating some routes for ruby synthesis, and ruling out the Czochralski process, some other process involved the use of a non
specified solvent that would create a big bubble in the final product.
Thanks again for your attention
PS: Don't mind the costs of these operations.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Have you looked into the Verneuil process? It's the classic method of ruby synthesis. No solvents are needed but you need sodium-free powderedalumina, oxygen and hydrogen
sources, and careful control of (very high) temperatures. Crystals can grow to about 25 grams.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
If you cannot acquire pure alumina, I would recommend synthesizing aluminum hydroxide. This will decompose to pure aluminum (III) oxide and hydrogen
when exposed to high heat. However I have heard that bubbles form in the boules when Al(OH)3 is used, probably due to hydrogen gas escaping the melted
mass.
"Is this even science anymore?!"
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TheAlchemistPirate  | | If you cannot acquire pure alumina, I would recommend synthesizing aluminum hydroxide. This will decompose to pure aluminum (III) oxide and hydrogen
when exposed to high heat. However I have heard that bubbles form in the boules when Al(OH)3 is used, probably due to hydrogen gas escaping the melted
mass. |
Did you mean water? Hydrated alumina releases steam on calcination, not hydrogen.
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by TheAlchemistPirate  | | If you cannot acquire pure alumina, I would recommend synthesizing aluminum hydroxide. This will decompose to pure aluminum (III) oxide and hydrogen
when exposed to high heat. However I have heard that bubbles form in the boules when Al(OH)3 is used, probably due to hydrogen gas escaping the melted
mass. |
Did you mean water? Hydrated alumina releases steam on calcination, not hydrogen. |
I meant that aluminum hydroxide (not aluminum (III) oxide) releases hydrogen gas on calcination to form Al2O3, which is what is of course the desired
chemical in corundum gemstones. I figured that maybe the aluminum hydroxide might not decompose completely in the flame and continue decomposing in
the molten boule, forming bubbles.
"Is this even science anymore?!"
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by TheAlchemistPirate  | Quote: Originally posted by blogfast25  | Quote: Originally posted by TheAlchemistPirate  | | If you cannot acquire pure alumina, I would recommend synthesizing aluminum hydroxide. This will decompose to pure aluminum (III) oxide and hydrogen
when exposed to high heat. However I have heard that bubbles form in the boules when Al(OH)3 is used, probably due to hydrogen gas escaping the melted
mass. |
Did you mean water? Hydrated alumina releases steam on calcination, not hydrogen. |
I meant that aluminum hydroxide (not aluminum (III) oxide) releases hydrogen gas on calcination to form Al2O3, which is what is of course the desired
chemical in corundum gemstones. I figured that maybe the aluminum hydroxide might not decompose completely in the flame and continue decomposing in
the molten boule, forming bubbles. |
No, it doesn't release hydrogen. It releases water.
2 Al(OH)<sub>3</sub> --> Al<sub>2</sub>O<sub>3</sub> + 3 H<sub>2</sub>O
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TheAlchemistPirate  |
I meant that aluminum hydroxide (not aluminum (III) oxide) releases hydrogen gas on calcination to form Al2O3, which is what is of course the desired
chemical in corundum gemstones. I figured that maybe the aluminum hydroxide might not decompose completely in the flame and continue decomposing in
the molten boule, forming bubbles. |
I don't know where you get that idea from. ALL hydrated metal oxides ('hydroxides' if you prefer) on calcination dehydrate to the corresponding
anhydrous oxide and steam (water). Hydrogen simply doesn't come into it al ALL.
In some rare cases the metal may undergo further oxidation but even then hydrogen is not a factor.
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
Apologies,
It seems I have screwed this one up. I was researching this earlier and thought a paper claimed it produced hydrogen, but when I look now it produces
water. When you referred to hydrated alumina I thought you meant aluminum oxide wet with water in some way  . I have never heard of referring to a (insert metal) hydroxide as a hydrated metal oxide before now. Anyways, it
should still form Al2O3 if you use Al(OH)3 instead. . I have never heard of referring to a (insert metal) hydroxide as a hydrated metal oxide before now. Anyways, it
should still form Al2O3 if you use Al(OH)3 instead.
"Is this even science anymore?!"
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | Have you looked into the Verneuil process? It's the classic method of ruby synthesis. No solvents are needed but you need sodium-free powderedalumina, oxygen and hydrogen
sources, and careful control of (very high) temperatures. Crystals can grow to about 25 grams. |
Yes I have, but I don't have that kind of apparatus. Moreover, as I specified before, the maximum I can reach is 1900°C.
I've already read the current methods to prepare it, I'm asking if it would be possible to achieve the same results by using the tools I've listed,
those being:
1) Hot Pressing machine that can reach up to 1900°C and 30MPa
2) Furnace in air that can reach up to 1200°C
I have pure Al2O3 and Cr2O3, so the starting material is not a problem.
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I get lots of hits when I google for 'hydrothermal sapphire' (or ruby)
or 'flux method corundum'.
Quickly scanning there seem to be texts among them that give practical details. I can't be bothered to read all of it to find out if it answers your
questions, but are you sure you have read the existing literature well?
I found at least one reference to tungstates being used as a flux for instance. And the hydrothermal method seems to have been common for some time to
produce artificual gemstones. eg. Laudise et al JACS (1958): Hydrothermal Synthesis of Sapphire (http://pubs.acs.org/doi/abs/10.1021/ja01544a014?journalCode=...)
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
chornedsnorkack
National Hazard
   
Posts: 521
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Corundum is stable in water above 360 Celsius and 170 bar:
http://www.minsocam.org/ammin/AM57/AM57_1375.pdf
At low pressure, the standard solvent for Al2O3 is cryolite, Na3AlF6. Pure cryolite melts at 1012 degrees.
Addition of solutes lowers the temperature to about 950 degrees, where alumina is normally electrolyzed.
Pure NaF would melt at 993 degrees.
Would it be enough to digest finely dispersed alumina in 950 degrees molten cryolite - does corundum readily have rapid crystal growth and rejection
of impurities at these conditions?
If you want to force supersaturation and alumina precipitation, can you form it out out cryolite? Like
2Na3AlF6+6NaOH->12NaF+Al2O3+3H2O
2Na3AlF6+3Na2CO3->12NaF+Al2O3+3CO2
?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. If memory serves. Hydrothermal crystal growth of this type, is best accomplished, via using Vernuil "Ruby" Boules as a feedstock.
Seems as if un-fused powders produce inferior results.
Same hydrothermal principles might be applied to growing from a melt. Though the viscosity of the molten solvent, might prevent effectively using a
temperature gradient and convection currents, to transport and recrystalize gem material onto a seed.
Also of note.....These melts, and crystalizations are sometimes conducted inside of sealed Platinum crucibles.
Could be better ways. Lots of general information available. Not much explicit detail.
Propietary information. Them whut a knows how, ain't a willingly tellin'.
Best damned Emeralds I ever saw were Gilson's. Magnificent!
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
So I'm new to the group as of today. I've been a creeper onlooker for the past five months. 
I know the storm that will follow shortly after announcing this claim I about to unleash. My name is Matt Wilson. I am an engineer by trade for 20
years. My current Bobbie is making synthetic ruby and sapphire. I make my own high temp ceramics created by my own designed techniques. Which
supports aspects of the Verneuil furnace I am designing building. To this point I have only performed tests on various concepts using crude methods
per use of a MAPP/OXY torch. No these are not gem quality crystals or even good enough for industrial applications. It is however perfect for creating
infant boules to use as seeds in other methods. They will work for beginning a flame-fusion crystal. You can study the crystal lattice under the
microscope and study the color of your samples to master the chemistry. There are ways to deal with hydrocarbon contamination.
I am just laying down for a bit before working all night, but I have seen only a few forums of chemist types, engineers and academic types always
shoot down a person that wants to try doing it, so they lay by the weigh side without trying and agree on points brought to them to discourage them
from ever even trying. Subconscious thing to fit in or are embarrassed to speak up for fear of what is and should be expected. Critics. Critics may
come as harsh and in numbers. This is an opportunity to take notes and learn some new tricks, it is also opportunity to teach those whom dismiss you
and make you feel embarrassed. This is when I provide to them answers to obtain through questions. Doubt my processes and evaluate my procedures and
critique with total brutal honesty.
Since I need to rest for a few hours before working tonight, I wanted to rejuvenate others interested in creating synthetic flak fusion ruby,
sapphire, corundum, futile. Troll all you want during this nap of mine. Tonight I will update this thread with pictures, videos and perform
demonstrations of my techniques for growing crude infant boules. There is so much info to be obtained by experimenting with what you have available,
study it under a microscope and find similarities in lattice structure between your homemade crystal, good quality synthetically produced crystal and
natural crystal. I have it all and will show you how they are alike in every way. The difference is, my boules won't grow from my crude method to
perform tests, but once my Verneuil furnace is complete I will be growing clean boules into larger and larger crystals.
I will write down all my data for others to have a chance to reproduce what I have done and a chance to publically humiliate me for being some sort of
fraud. Call me out for whatever, but I can show everyone how to make real synthetic ruby and sapphire. I will give my formulas and explanations why
the cbemical formulas I have created work the way they do.
I will detail all my failures too. I hope to encourage those that once thought about doing this on their own, to pick up some oxides, proper hydrogen
oxygen split cell apparatus, firebrick and a few other things. I just have been doing this for over a month and a half, but I can share my methods,
formulas and everything else I have to help others to do this on their own. I'm not selling my way of doing things in a book or promote any products.
I want this info freely available since there is a niche of people interested in creating homemade corundum. I want a member count to make amateur
ruby and sapphire makers to meet up online and the real world and turn this into a legit hobby of sharing information with people all over the world
like Locksport.
I will be back on later tonight, post links for my YouTube channel with a few videos on this subject. I have a shared Google+ album with my homemade
corundum. I document everything I do so I can pass that info on.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
https://photos.app.goo.gl/ZhLsxp9B5NDRjsIV2
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
My YouTube video showing more on synthesizing ruby.
https://youtu.be/pIA67ZFeiS8
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here is a picture of just one of the boules I created. The lattice structure is more apparent on the last part of the boule to touch the flame.--the
newest crystal on the surface. I'm going to be gathering more pictures since I've cleaned up a lot of the boules in several acids and bases. I've
found a great Sierpinski triangle structure in one of the sapphire boules I made, but I need to re-find it and get a picture with my microscope
camera.

|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
LOL. I hate my username. I need to change that.
Anyhow, I'm not trying to bump my post, but I'm wondering if anyone else has tried to produce crude corundum and created any successful sapphire
formulas. Ruby has a very low tolerance, so it's actually pretty easy to create something from crude caveman like labs. Sapphire on the other hand
is not anywhere near as forgiving. I do have a formula that seems to work for dark blue crystals in a reducing flame. I imagine more impressive
results with an oxy-hydrogen flame in a Verneuil furnace.
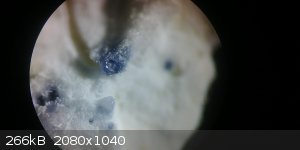
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Yeah, I know it's microscopic, but it's only to test experimental formulations for sapphire. I've got some specimens which are covered in rutile, or
what I perceive as rutile.
[Edited on 17-4-2018 by Doped-Al2O3-fusion]
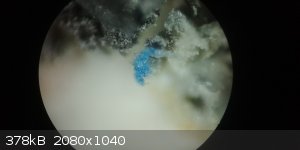
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
This was from my first experimental run of ruby. It exhibits the same crystal lattice as seen on other synthetic specimens from Djeva and from
natural corundum. I'm hoping to have my Verneuil furnace completed by this summer.

|
|
|