AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
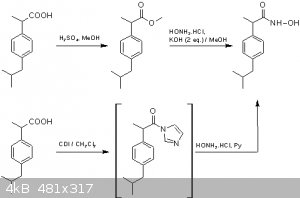
Ibuprofen - Methyl ester and hydroxamic acid derivatives
2-(4-Isobutylphenyl)propionic acid, or ibuprofen, is a readily available organic compound which can lend itself to many reactions by the interested
home chemist. For some exploratory chemistry I wanted to undertake I needed a phenylacetic acid type of compound readily available in large amounts
from which I could make its hydroxamic acid derivative. Ibuprofen, which can be considered an alpha-substituted phenylacetic acid, fit that
requirement and its hydroxamic acid derivative is a known compound as well [1].
Hydroxamic acids are classically prepared by the reaction of an ester with hydroxylamine under carefully controlled conditions. The methyl ester was
chosen as the staring point for this chemistry as it turns out to also be a known compound presumably as the (+/-)-racemate [2]. Ibuprofen was
isolated from OTC tablets using a simple extraction method [3]. It was assumed that the ibuprofen was the racemic mixture which was consistent with
the observed melting point (74-77 C). The ester was prepared by treating a methanolic solution of ibuprofen with sulfuric acid for several hours at 50
C followed by a standard workup. The isolated ester was then purified by vacuum distillation. However, the undistilled ester is probably pure enough
for most uses.
It was later found that the ibuprofen did not need to be isolated as a separate step prior to ester formation. The powdered tablets were stirred with
methanol and the mixture was filtered under vacuum through a pad of diatomaceous earth. The filtrate was treated with sulfuric acid and the
esterification carried out as normal. This is very expedient if large quantities of the ester are required.
The reaction of the ester with hydroxylamine was based on a published procedure in Organic Syntheses [4]. The procedure needs to be followed quite
carefully for any measure of success. It is important to use two moles of potassium hydroxide per mole of hydroxylamine hydrochloride. If only a 1:1
molar ratio is used, there is only a small yield of hydroxamic acid formed as hydroxylamine itself is a relatively poor nucleophile. The reactive
species in the reaction with esters is not hydroxylamine but the much more nucleophilic hydroxylamine anion:
NH2OH + KOH -> NH2OK + H2O.
The pKa of the OH proton of hydroxylamine has been reported to be 13.7 [5] and is therefore easily removed by KOH.
The hydroxamic acid can be more conveniently synthesized via an activated carboxylic acid and hydroxylamine. The imidazolide of ibuprofen, formed by
reaction of ibuprofen with carbonyldiimidazole (CDI) is such an activated species. Although several examples of this type of procedure exist, recently
DFJ90 posted a paper illustrating the reaction [6].
Utilizing either the ester or the imidazolide, I was able to secure what I believe to be the hydroxamic acid. I say “believe” because my product
exhibits a melting point higher than that reported [1]. My compound shows a melting point of 127-129 C independent of synthesis route. The compound
gives a very positive test with FeCl3. My thought is that both of the reported melting points are for the S-(+)-isomer and that I have the racemate.
It is interesting that the S-(+)-isomer of ibuprofen itself melts about 50-52 C while the racemate melts at 75-77 C. [7]
Overall, the use of CDI, though somewhat expensive, is a more convenient way to obtain the hydroxamic acid. However, with appropriate care, the ester
also works acceptably well though it does require a large excess of hydroxylamine hydrochloride.
Experimental
All chemicals and solvents were purchased from known, reputable sources with the exception of pyridine which was a gift. Solvents were distilled if
required. Melting points were determined in capillaries using a Mel-Temp apparatus.
Methyl 2-(4-Isobutylphenyl)propionate (Ibuprofen methyl ester)
Purified ibuprofen (10.31 g, 50 mmoles, mp 74-77 C) was dissolved in methanol (100 ml). Sulfuric acid (10 ml) was added drop wise followed by
immersing the reaction flask in a 50 C bath. After 5 hours, the reaction was cooled and added to 6N sodium hydroxide (50 ml) diluted with ice water
(500ml). The aqueous mixture was extracted with hexanes (3x50 ml) followed by washing the combined extracts with water (50 ml) and brine (50 ml) then
drying over sodium sulfate. The hexane solution was filtered and concentrated by simple distillation to a clear, mobile oil (12.12 g, theo yield:
11.01 g). The crude product was distilled under vacuum using a short path 14/20 system to afford 10.07 g (91%) of pure product, bp 97-99 C @ 1.2 mm.
Single spot by tlc: Rf 0.66, silica gel, 9 hexanes:1 ethyl acetate.
Alternate preparation: Ibuprofen tablets (100 tablets containing 200 mg ibuprofen each, ca 20 g ibuprofen) were ground to a powder. The powder was
added to methanol (200 ml) and the mixture stirred with gentle heating. The suspension was then filtered through a pad of diatomaceous earth (food
grade material) with gentle vacuum. The solid cake was washed with methanol (20 ml). Concentrated sulfuric acid (20 ml) was added drop wise to the
filtrate and the reaction carried out as described above. The crude ester from the hexane extracts (19.87 g, theo yield 21.35 g based on 100 tablets)
was distilled to afford 18.69 g (88%) of pure ester.
N-Hydroxy-2-(4-isobutylphenyl)propionamide (Ibuprofen hydroxamic acid)
From the ester: Hydroxylamine hydrochloride (4.20 g, 60 mmole) was added to methanol (22 ml) and chilled in an ice bath. Potassium hydroxide (food
grade flakes, 6.72 g, 120 mmole) in methanol, likewise chilled, was added slowly to the hydroxylamine solution affording a thick white precipitate.
The cold mixture was quickly filtered under mild vacuum. To the cold filtrate, ibuprofen methyl ester (4.40 g, 20 mmole) in methanol (4 ml) was added
and the clear reaction mixture transferred immediately to a preheated 60 C bath. After about 15 minutes, tlc (see above) showed complete absence of
ester and a single spot at the origin. A microdrop of 5% ethanolic FeCl3 solution gave an intense red color at the origin indicating hydroxamic acid.
The reaction was cooled to room temperature and acidified to congo red with 10N hydrochloric acid. The mixture was chilled in the refrigerator
overnight and the resulting solids isolated (5.2 g). The solids were taken up in methylene chloride (50ml) and the mixture dried over sodium sulfate.
After filtration, the solvent was removed by simple distillation until the first sign of crystallization appeared. The pot mixture was chilled to room
temperature then treated with hexanes (20 ml). The solid was transferred to a funnel and washed with hexanes (ca 10 ml) then air dried (2.93 g, 66%,
mp 123-125 C). Recrystallization from acetone-hexanes gave a nicely crystalline white solid, mp 127-128 C. The ferric chloride test was positive.
From the acid using carbonyldiimidazole (CDI): Purified ibuprofen (2.06 g, 10 mmole) was dissolved in methylene chloride (20 ml). Under good stirring,
CDI (1.64 g, 10 mmole) was added in a single portion resulting in vigorous evolution of carbon dioxide. After ca 20 minutes, hydroxylamine
hydrochloride (0.80 g, 11.6 mmole) was added followed by pyridine (1 ml, ca 12.7 mmole). [NOTE: Not everyone has access to pyridine. See Ref 6 below
for alternate method.] After ca 8 hours the reaction was quenched by the addition of 2N hydrochloric acid (25 ml). The organic layer was separated,
washed with saturated sodium bicarbonate solution (ca 20 ml) followed by 50% saturated sodium chloride solution then dried over sodium sulfate. The
solvent was removed by simple distillation at a bath temperature of 75 C. When nearly all the solvent was removed, the pot residue solidified. The
solid was triturated with hexanes (ca 10 ml) and isolated (1.58 g, 71 % crude, mp 118 – 124 C). A second trituration with warm hexanes (10 ml) gave
a white solid (1.43 g, mp 127-129 C, 65%) which gave a strong FeCl3 test. Acidification of the bicarbonate wash gave a yellowish crystalline solid
(0.45 g, mp 68-72 C) which was impure recovered ibuprofen.
References
1. The hydroxamic acid derivative is known as ibuproxam and is listed in the Dictionary of Pharmacological Agents, 6th Ed., 1995, as entry I-00013.
It is also described in several patents including: GB 1447658 and US 5840714. The GB patent seems to describe the (+/-) - racemate while the US patent
seems to describe the S-(+)-isomer. Both report the same melting point (119-121 C).
2. Y. Tamura, et al., Chem. Pharm. Bull. Japan, 33, 1097-1103 (1985). The boiling point is reported 107-108 C at 5 mm. Methyl ester CAS 61566-34-5.
3. Source unknown, see pdf attachment.
4. Organic Syntheses, Coll. Vol 2, p 67.
5. M. N. Hughes et al., J. Chem. Soc. A, 1971, 3485-3487.
6. J. Zhao, et al., Org. Process Res. Dev. XXXX, XXX, XXX−XXX, DOI: 10.1021/op500379a, http://www.sciencemadness.org/talk/viewthread.php?tid=65413
7. Dictionary of Pharmacological Agents, 6th Ed., 1995, entry I-00012
Attachment: Isolation of ibuprofen and naproxen.pdf (26kB)
This file has been downloaded 3433 times
[Edited on 29-2-2016 by AvBaeyer]
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Excellent work AvBaeyer! I have been wondering about Ibuprofen derivatives for a long time, and you have opened my eyes to a variety I wasn't familiar
with 
nlegaux
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I'd like to congratulate you on an exceptionally clear and well written report. I like how you confirmed the successful formation of the hydroxamic
acid by using two different synthetic approaches and obtaining identical material from each (the only thing missing is a mixed melting point), and the
use of a chemoselective TLC stain. Maybe this should be moved to Prepublications? It's certainly of a suitable caliber.
Any pictures?
Note: That OPRD paper should have been assigned page/volume numbers by now, its just that the copy I had saved at the time was an ASAP article.
[Edited on 29-2-2016 by DJF90]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A very good report. Thanks for sharing. I'll just add below the scheme, so that those less versed in organic synthesis understand what it is about.
I agree that this should be moved to Prepublication. Unless you have objections I will do so once the discussion settles down.
| Quote: | | It is important to use two moles of potassium hydroxide per mole of hydroxylamine hydrochloride. If only a 1:1 molar ratio is used, there is only a
small yield of hydroxamic acid formed as hydroxylamine itself is a relatively poor nucleophile. The reactive species in the reaction with esters is
not hydroxylamine but the much more nucleophilic hydroxylamine anion: |
Interesting. I did not know this, but it explains why I could not get a hydroxamic acid from a methyl ester when I tried to use potassium carbonate
and hydroxylamine hydrochloride in methanol. All that theory about the alpha-effect and then it still gave only traces of the product. I wonder why
hydrazine works normally?
| Quote: | | [NOTE: Not everyone has access to pyridine. See Ref 6 below for alternate method.] |
It may not even be necessary to use an additional base, except for improving yields, because there is already one eqiuvalent of imidazole present as
the by-product from the activation step with CDI. Imidazole, pyridine and hydroxylamine are similarly basic. Alternatively, triethylamine could be
used, or less preferably potassium carbonate with THF or acetonitrile as the solvent (though acetonitrile is not inert to hydroxylamine).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Well done.
You could write a page on smwiki about it.
Or if you agree, I can copy and paste it there and write you as an author.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2659
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That writeup is better than most of the papers I reference in daily work, and many of my peers lab notebooks. Nice job.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
First, thanks to all for the nice comments. I did an edit earlier to clean up some typos and grammar problems.
Responses:
nlegaux: Have at it! Both ibuprofen and naproxen provide potential for some interesting organic chemistry.
DFJ90: Regarding photos, I am not really up to that. The time I spend taking pictures and figuring out what to do with them takes time away from
washing glassware. My wife keeps telling me to clean up because "You don't have people anymore."
Nicodem:
1. It's ok to move this post if you think it's justified. I have a few more write-ups in the works involving chemistry that can be done with ibuprofen
and related compounds. Just a matter of time.
2. I think that the reactivity difference between hydrazine and hydroxylamine towards esters resides is the greater e-withdrawing power of oxygen vs
nitrogen which renders the amine function of hydroxylamine less reactive. Perhaps this is supported by the weaker basicity of hydroxylamine compared
to hydrazine.
3. I tried other bases in the CDI reaction. Triethylamine works fine. I chose pyridine because I felt it might form a transient acylpyridinium species
with enhanced reactivity. I also used N-methylimidazole and the reaction was over as fast as I could spot a tlc supporting the notion that an
N-acyliminium species may be formed. I did not report this as I don't think most people have access to N-methylimidazole. I did not try DMAP and I did
not run the reaction in the absence of any added base.
4. Thanks for posting the flow chart. Can you tell me how to get these diagrams into a post (I am just an old fart.) I have ChemDraw and IsisDraw at
my disposal.
Crystal grower: Fill me in on what the wiki is all about.
Dr Bob: I have had my share of hammering by bosses and referees over the years. I guess some of it stuck.
AvB
[Edited on 1-3-2016 by AvBaeyer]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
@Av - If you have more work planned with Ibuprofen then perhaps (if Nicodem and yourself agree) it can all be compiled into a single "chemistry of
ibuprofen and derivatives" type thread in prepublication.
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
@AVbaeyer The Science madness wiki is official wiki of science madness.
It is meant to be a repository of information that is useful to home chemists. It is edited only by members of the Sciencemadness Discussion Board,
rather than being open to the public, meaning it is guaranteed to be a collaborative work of home chemists from around the world. Here you can find
descriptions and pictures of many chemical compounds, write-ups of procedures developed by Sciencemadness members, and much more.
(If you want become an editor of smwiki just send u2u to zts16).
Here's a link to sciencemadness wiki:
http://www.sciencemadness.org/smwiki/index.php/Main_Page
Cheers.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AvBaeyer  | | 1. It's ok to move this post if you think it's justified. I have a few more write-ups in the works involving chemistry that can be done with ibuprofen
and related compounds. Just a matter of time. |
OK, then we'll wait for more before making it an official Prepublication thread.
| Quote: | | I did not report this as I don't think most people have access to N-methylimidazole. |
You never know. Since some people get some of their chemicals as decommissioned goods from various labs, it is often the case that one obtained a
certain exotic reagent, but can't get something trivial. Besides, it is always interesting to learn new things from someone's experience. I mean, it
is not just a procedure to make some product, it is an experience to learn from.
| Quote: | | 4. Thanks for posting the flow chart. Can you tell me how to get these diagrams into a post (I am just an old fart.) I have ChemDraw and IsisDraw at
my disposal. |
It's easy. You just create whatever scheme on whichever software you use (I generally use either ChemDraw or ChemSketch, depending on which computer I
work). Next, you save it as a GIF picture and upload it in the post using the "Attachment:" field below the text box, using the "Browse ..." button to
locate the file. All of these drawing softwares have an export option to save the schemes as pictures (GIF, JPG and PNG formats are tolerated by all
the Internet browsers).
If you only want to show a single molecular structure, it is even easier. You just insert its SMILES code in between a "[smiles ]" and "[/smiles ]"
tags (without the blank character). For example, writing [smiles ]CC(C(=O)NO)c1ccc(CC(C)C)cc1[/smiles ] gives:

Or you can use trivial names directly, like in [smiles ]ibuprofen[/smiles ] which gives:

For more info on this forum code, see this thread.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemplayer..
Awesome

Posts: 48
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
Thanks very much for this. We're looking at doing some reactions with ibuprofen as well, but this is one we hadn't heard of so learned a lot from
reading this and looking up the reactions.
|
|
|
|