quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
halo-formation/genation control/selectivity and explosive hazard!? haloform and halogenation thread (possibly)
https://www.sciencemadness.org/whisper/viewthread.php?tid=75...
PHILOU Zrealone >>>" posted on 5-3-2003 at 01:38
To allow good reaction to make halocetons
ICH2-CO-CH3, BrCH2-CO-CH3 and ClCH2-CO-CH3, you need catalytic amounts of acids...HCl!
They were registred as XCH2-CO-R
There is serious risks with the above chemicals because they tends to polymerise explosively in large quantity and spontaneously upon time!
"
i need to know the safety/hazards of this. this seems to mean iodoacetone is explosive on standing from reacting/polymerizing or from the unstability
of the polymer.!
!!!!!!!!!!!!!!!!!!!!!!!^^^^^^^^^^^^^^^^^^^^^^^!!!!!!!!!!!!!^^^^^^^^^^^^^!!!!!!!!!!!!!!!!!!!!!!!^^^^^^^^^^^^^^^^^^^^^^^
i have saved, a reaction of iodine with acetone and copper/CuI with no hydroxide and UV
and
an attempt at making sodium cinnamate, with cinnamaldehyde with iodine and UV (and i believe sodium hydroxide)
they both are interesting outcomes, over a year or maybe two ago.
the cinnamon one is in a 5ml round bottom with 2 layers, i have been curious to reapproach it.
the other thing was smelly and i am curious about it, although the smell seems to be less now.
i am hoping to produce experiments along these lines and produce different halogenated methanes for curiosity.
so please let me know what i can do to avoid explosive reactions, also to obtain pure results, and lastly, where to find an actual chemical properties
database (m.p, b.p, density@temp)
i originally sought/seek how to run a halogenation selectively
1. iodoacetone
2. diiodoacetone
without a leftover amount of acetone or contamination from the other.
because i don't know if it is easily separatable by distillation
in the case of iodoacetone
well here is what i think of
1. stochiometric amounts of iodine and acetone would in reality, probably leave the reaction unfinished.
2. more acetone than iodine would possibly make an azeotrope with the iodoacetone, making seperation by distillation tough.
3. adding exess iodine might over halogenate the acetone, leaving diiodo and iodo acetones mixed and perhaps difficult to separate .
4. assuming that a pH threshhold is needed for diiodoacetone, a weakly acidic solution may allow only iodoacetone; however, on distilation, the
diiodoacetone might then form.
5. a catalyst may help the reaction,
according to
https://www.sciencemadness.org/whisper/viewthread.php?tid=18...
Quote: Originally posted by neptunium  | i also should point out that instead of HCl i was going to use CuO ... i red somewhere that it was a better yield and accelerate the reaction ..
[Edited on 19-1-2012 by neptunium] |
; however, what are it's effect on the degree of halogenation, like the previous question; furthermore, what of forming other products on
distilation....
(from this paper which i can't access, and which is photocatalyzed; however, the fact that it uses a catalyst in general is what has me inquiring
(although light is seemingly not a technical catalyst, being used up in the reaction).
http://link.springer.com/article/10.1134%2FS1070428008100278
"Photochemical disproportionation of 1-iodoacetone. New method of synthesis 1,3-diiodoacetone" which gives regular acetone and the 1,3 kind after
disproportionation.
and the availability of melting, boiling points, azeotropes and densities at different temperatures, are still elusive to me. having a mathematica
program equivalant for chemistry, for free on the rasberry pi would be great.
lastly, what are the effects of these compounds on the halogenation of acetone with regards to pH?
Zn (low acidity)
ZnS
CuS (to make a comparison to CuO)
Pt
Ni (low acidity)
Pd
V2O5
CuI
i am wondering about electrochemistry too; however, the questions i asked are what i am mainly stumped on for now, as i am hoping to learn, goal wise,
halogenation/haloformations of ketones.
i am hoping to form a pure halogenated ketone, mainly iodoacetone;
and then after seperation/purification, halogenate it;
with NaOCl and NaOH, to maybe get iododichloromethane / dichloroiodomethane / CHICl2
;and then also try it with diiodoacetone to get diiodochloromethane / chlorodiiodomethane CHCl2I
i also want to get a decent idea of catalytic behaviour from different compounds dependant on pH, i feel confident that would give me things to muse
over.
i tried some halogenation experiments and one of them was with iodine in acetone and UV.
i tried UV on iodine and copper in acetone and i don't remember if i added CuI after that and ran UV more.
i did some tinkering and i didn't document it. i want to revisit it and have to go back and retroubleshoot it, which is part of my reason for
questioning now. if there is a polymerization explosion hazard, i need to know about it.
one reaction i got was odd as it didn't tear, but smelt real wierd.
i only noticed tearing in another reaction with combination of aluminum foil in basic acetone with iodine. i was hoping it was COI2 and realized it
was seemingly iodoacetone.
with hypochlorite/hydroxide and having the mixture touching aluminum, it seemed phosgene was formed, not chloroacetone because i think it had a
"field" type of smell, it burned my lungs and i think it may have made my eyes sting/water.
CHICl2, dichloroiodomethane (m.p=? b.p=? density @ various temperatures=?)
(1/3 haloform with iodine and then finished with bleach)
i guess that stoichiometric amounts of iodine and acetone could be mixed with hydroxide solution and then bleach added to haloform to completion. i
want to get an understanding from the acidic side of it though, with the pH and catalyst (metal/metal calcogenide) effects.
so, while i will probably try all sorts of stuff, i just want to get the data that is already there so i can focus my experiments. i don't have any
IR, mass spectrophotometer, or NMR or have learned how to build them yet, so i am relying on the sciencemadness community for already known chemical
knowledge. i may build an IR machine soon if i can learn enough about it, but that is going to take some time.
[Edited on 5-3-2016 by quantumcorespacealchemyst]
[Edited on 5-3-2016 by quantumcorespacealchemyst]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
*sigh*
Just do an experiment then let's talk about the results of That maybe ?
Chemistry is a strange thing. 99% theory, speculation, calculation, yet that last 1% of actually Doing Experiments is the thing that drags in the
interest.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
ok
i want to try the iodination of acetone first, but i will need some acetone (and bleach to bring it home to a halomethane).
while
i have the two reactions from 1-2 years before, (which i fixed on the last edit) which i am most interested in the cinnamon one. the other one seems
relevant to disproportionation.
i can maybe post the picture of the phase separated cinnamonaldehyde experiment, in the meantime
i originally thought aldehydes and ketones could both be haloformed and after finding out it was only acetaldehyde and ketones part way into the
experiment, i figured id see if prolonged UV would help. i put it on hold, hoping to get back to it, (and more cinnamaldehyde) and noticed there was a
phase separation.
i will post up pictures when i can charge my camera.
the polymeration and explosiveness of iodoacetone is troubling though. i did not expect that but more so, i am stressed to not have the information.
[Edited on 6-3-2016 by quantumcorespacealchemyst]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I can make no sense of the words posted.
This is all beyond my knowledge.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Acetyl iodide's boiling point is 108 C and I have to assume iodoacetone's is even higher. Acetone by contrast boils at 65 C so I think forming an
azeotrope won't be too much of a problem. Aga is right though: nobody can say for sure unless they've done the procedure, and iodoacetone is bound to
be a nasty fucker so it's probably not too popular of a reaction.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
googling gave me 163.1C plus or minus 23C at 760mmHg from "predicted ACD/Labs Percepta Platform Phys/Chem module" i don't know about it's accuracy,
for example, the flash point is listed as 52.4C plus or minus 22.6C. facepalm
the program is accessed by google it seems but it also seems the program isn't free. if it is, i also don't even know if it is accurate.
the boiling point of CHCl2I, dichloroiodomethane is listed 128C at 760mmHg at one page and 132C without a pressure mention at another place. i also
seem to keep finding density measurements without a temperature mention, in both compounds searched. it boggles my mind. how did chemists do anything
with such piss poor access to data, and, when they didn't even have the internet.? i guess its out there.
i guess the b.p is about right in the case of CHCl2I, i wonder if it is a better solvent than chloroform, but with a higher boiling point. around
128C, which is still lower than DMSO, which i googled to recheck it's B.P
and......
off topic:
the way my mind is going, i saw a picture of dmso again and my mind started wondering if it could be haloformed (being a sulfonyl version of acetone,
(if acetone is a carbonyl. i mean the carbon middle is a sulfur, same molecule....almost)). i read a theory it turns to dimethyl sulfone with bleach,
possibly. the best source i found was yahoo answers in what happens mixing dmso and bleach. :/ , and somewhere that it can go from msm to dimethyl
sulfate with more oxygen, but with the structural differences, and my current amount of knowledge, it doesn't seem to be what would happen. i am
wondering if a sulfonyl (right word?)acid salt could be made in a haloform reaction. because, if the bleach tacks on an oxygen with double bond to
dmso, then the haloform mechanism continues...maybe it can trichlorinate the methyl group, cleave it and leave a sodium salt of the rest.
i don't know when i'll get the money to procure the chemicals and find the conditions to run the experiments in. hopefully soon. the storage life of
iodoacetone is what though? explosive polymerization? is this the kind that
PHILOU Zrealone means
https://www.youtube.com/watch?v=h4pNXAtPJp8
?
[Edited on 6-3-2016 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
i think i found a description about the polymerization
a_bab
"The bromacetone life is about 1 month. It'll polimerise slowly and turn into a black slug (like when the phenol is oxydised by the nitric acid in a
failed TNP synthesis, a few of us know what I mean). I read that is possible to stabilise it by the means of MgO."
https://www.sciencemadness.org/whisper/viewthread.php?tid=75...
i wonder why it does that
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
picture of the cinnamaldehyde attempt
i don't recall the reaction completely now. i tried few-several attempts to haloform cinnamaldehyde (i read wrongly? aldehydes and keytones could be
haloformed. i thought i would get sodium cinnamate, which is a photoswitchable hydrotrope, which seems useful for odd reactions).
http://www.sciencedirect.com/science/article/pii/S0167732211...
i did try adding acetone in one reaction, i don't think it was this one.
there was iodine, cinnamaldehyde, sodium hydroxide (in some water i think, not much though, only enough to dissolve, i believe. this is hazy in
memory) and possibly CuI added. there are uncertainties here but nothing that couldn't be retraced by experiment.
anyway, there is a grey ring around the inner edge of the top brown/red layer. the amber layer is below and there are some salts deposited on the
bottom.
the septum has turned a slight brown tint over the year to two years. it smells like cinnamon 
    
[Edited on 6-3-2016 by quantumcorespacealchemyst]
[Edited on 6-3-2016 by quantumcorespacealchemyst]
[Edited on 6-3-2016 by quantumcorespacealchemyst]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Cinnamaldehyde cannot undergo the haloform reaction. Only methyl ketones and acetaldehyde will.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
do you think the cinnamaldehyde was altered on the hydrogen connected to the carbonyl, a carbon on the chain or on the ring, perhaps on the meta or
para position. maybe it cleaved somewhere? do you think it polymerized (my guess, no). the dark brown/red color was the product, when i stopped the
experiment. the amber color separated over time.
in general, what probably happened, instead of haloformation?
[Edited on 7-3-2016 by quantumcorespacealchemyst]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Most likely, iodine adds to the alkene giving 3-phenyl-2,3-diiodopropanal. This can polymerize by aldol condensations.
[Edited on 7-3-2016 by clearly_not_atara]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | https://www.sciencemadness.org/whisper/viewthread.php?tid=75...
" To allow good reaction to make halocetons
ICH2-CO-CH3, BrCH2-CO-CH3 and ClCH2-CO-CH3, you need catalytic amounts of acids...HCl!
They were registred as XCH2-CO-R
There is serious risks with the above chemicals because they tends to polymerise explosively in large quantity and spontaneously upon time! "
phil, you posted that there. i need to know the safety/danger of this, as i don't know about it and what that means.
like this?: https://www.youtube.com/watch?v=h4pNXAtPJp8
or makes a polymer that is explosive?
anyway, if i make and store iodoacetone AcCH2I, what do i need to be aware of?
also if i start to play with halogenating other parts of acetone (for different salts and haloform products), are there similar cases to watch for and
what?
thanks.
i figure i can run a reaction to get iodoacetone and then haloform the rest with bleach, but i want to be sure that all the safety data is known if i
want to store something. |
You post manyfold in various place of the forum about the same subject...try to stick in one place...
The explosive polymerization only occurs if in large quantity (>> 1L and thus on industrial scale)!
Your example is not really what I thougth because it is minor quantity and heated by a Bunzen burner...but it may go that way.
An explosive polymerization happens when the compound is in large quantity and confined...slow polymerization occurs and heat the batch slightly
favourizing more polymerization...while more polymer do form, the viscosity of the fluid increases thus reducing the heat dissipation by
convection...thus the polymerization can go runnaway and heat even more...if the batch is large enough the heat Inside can be very hot and cause
cracking of the polymer and pressure increase with potential bursting and explosio.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
oh. is the polymer an oil? is there further reactions that can be done, maybe with a catalyst in acid or electricity? will the benzene be able to be
cleaved as a 2,3 diodobenzene? and with some species forming from the propionyl part.?
is it possible to work backwards somehow to make 1,3 diiodobenzene?
there's a tyrian purple synthesis that starts from p-dibromobenzene but lists m-dibromobenzene as cleaner/more efficient/more expensive. i am curious
about making 6,6-diiodoindigo and/or 6,6-dibromoindigo.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If you do a trimerization cyclisation of iodoaceton you don't get diiodobenzene but triiodomesytilene...
Maybe time for you to read or reread organic chemistry basics?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
can someone write out the full reaction/partial reactions of acid catalyzed halogenation of Acetone? i am confused on somethings about it. i was
dreaming sometime last night/this morning that i might try using a weak lewis acid catalyst, aluminum iodide, from aluminum foil and iodine.
also, if you can recommend a superb book or books to focus on, that would be great. i use wikipedia hotlinks to correlate things, google and the
forum. i ordered a book that i saw in a google preview, that had a reaction to make 1,3-dibromo benzene (a more ideal starter for tyrian purple than
1,4 dibromo benzene), but the seller never sent it and i forgot the book title. i had downloaded some pdf files here and there across my variably
operating computers, lol; but i was not sure to take my time towards one specific read or reads. i was surfing through the old royal society papers
looking for inorganic salts though. it seems the most educating data was there. i once found a book that had all sorts of named reactions in organic
chemistry (it was in the title too, "named reactions......." or something). it was expensive for me but i set it in my mind to get it and learn all
the mechanisms. now i have been unable to find the title. i only remember it was a purple cover and a recent publication within a few years.
Edit(woelen): Removed some personal info on request
[Edited on 29-3-16 by woelen]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by quantumcorespacealchemyst  | | can someone write out the full reaction/partial reactions of acid catalyzed halogenation of Acetone? i am confused on somethings about it. i was
dreaming sometime last night/this morning that i might try using a weak lewis acid catalyst, aluminum iodide, from aluminum foil and iodine.
|
Halogenation of acetone in acidic media proceeds via the usual mechanism for alpha-halogenation under acidic conditions. The catalyst in these
reactions is usually an acidic solvent (aqueous acid solution, GAA, etc); however, lewis acids in the form of AlX3 will also work. But
unlike aromatic halogenations, which typically require a lewis acid to activate the halogen for nucleophilic attack by the aromatic ring, ketone
halogenations will proceed just fine without them, as the intermediate enols are much more reactive toward halogens.
Below are the mechanisms for the halogenation of acetone, both in acidic media as well with a lewis acid catalyst. I only bothered to draw the
mechanism for chlorination, but it's the same as the other halogens. For the lewis acid, I used AlCl3.
Chlorination in acidic solvents:
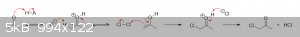
The above is basically your standard textbook mechanism for acid-catalyzed ketone halogenation. The H–A is the acid catalyst
(H3O+, AcOH, etc), and the A<sup>–</sup> is its conjugate base. The carbonyl oxygen is first protonated by the
acid to give an oxonium ion, increasing the acidity of the alpha-hydrogens through the inductive effect. The conjugate base then removes one of the
alpha-hydrogens to give an enol, regenerating the acid catalyst. As a chlorine molecule approaches the enol, the electron-rich double bond pushes
electron density away from the closest chlorine atom due to the electrostatic repulsion between the electrons of the bonds. This polarizes the Cl–Cl
bond and creates a partial-positive charge on one of the chlorines, which quickly gets attacked by the nearby nucleophilic pi-bond. The resulting
chloride ion then grabs the extra proton to give chloroacetone and HCl.
Note that the chloro group decreases the basicity of the adjacent carbonyl group by pulling electron density away from oxygen through induction.
Because the oxygen is now more electropositive than before, protonation a second time becomes more difficult. Thus the reactive enol intermediate is
less likely to form a second (and especially third) time, stopping the reaction primarily at the mono-chlorinated stage.
Chlorination using lewis acid catalysts:
Halogenation using a lewis acid more or less works the same way as shown above; however, the lewis acid can complex with both chlorine as well as
acetone, forming far more electrophilic intermediates. Below are the two most likely adducts:
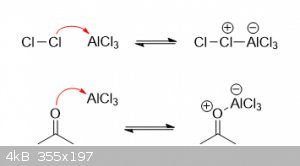
If the lewis acid complexes with a chlorine molecule, it will pull electron density away from the inner chlorine, greatly increasing the
electrophilicity of the outer chlorine in the process. If the lewis acid complexes with acetone, it will pull electron density away from the
alpha-hydrogens, significantly increasing their acidity and ease of removal. Depending on the exact conditions and solvent used (protic v.s.
aprotic), I imagine that halogenation would likely proceed through one of the following pathways. Note that the B: in the last two routes simply
represents anything with a free lone pair that could act as a base:
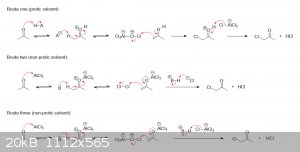
I hope this was helpful to you in some way. If you're still not clear on anything, just let me know.
|
|
|
|