electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Reduction at a Cathode
I'm trying to better my understanding of electrochemistry and there is a question I haven't been able to answer through research. It concerns the
priority with which cations are reduced at the cathode in an electrochemical cell when there is more than one species present.
For example, take a solution containing copper and silver ions. I want to plate out all the silver but leave the copper in solution. Assume these
are the only cations in solution. I insert two inert electrodes (graphite or Pt lets say) and apply a voltage to the solution. Which if any of the
following staements is true about what could happen at the cathode:
There could be a voltage/concentration combo at which all silver ions will be reduced, but never any of the copper ions.
There could be a voltage/concentration combo at which all the silver ions are reduced first, and then all the copper ions.
There could be a voltage/concentration combo at which both silver ions and copper ions are reduced at the same time, with the relative amount of
each depending on the exact voltage and concentration.
For whichever one or ones are true, how would I go about finding the voltage and concentration combination that would do the trick? Thanks for your
help.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
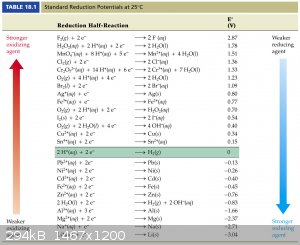
you need to read about this too
https://www.google.gr/#q=nernst+equation
[Edited on 28-3-2016 by gatosgr]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
oh shit-kinetics!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
So if I understand right, as long as the reduction potential for the silver is higher than that of the copper, after adjusting for concentration, then
the silver should plate instead of the copper.
|
|
|