Stibnut
Harmless

Posts: 43
Registered: 4-6-2015
Member Is Offline
Mood: No Mood
|
|
Oxidation of tertiary alcohol by KMnO4
I recently obtained some 2-methyl-2-butanol (aka tert-amyl alcohol), and I tried adding some potassium permanganate solution to a sample of it, with
excess alcohol. I was under the impression that tertiary alcohols should not oxidize, or at least not very quickly relative to primary and secondary
alcohols. But to my surprise it oxidized nearly as quickly as ethanol does, producing a brown MnO2 precipitate and turning the solution orange just as
ethanol and isopropanol do.
Does this indicate substantial impurities in the 2-methyl-2-butanol, or does KMnO4 actually interact with tertiary alcohols the same way as it does
with primary and secondary ones, or is there something special about 2M2B?
|
|
|
ch5
Harmless

Posts: 1
Registered: 7-4-2016
Member Is Offline
Mood: No Mood
|
|
No C-H bond, no oxidation. Must be contaminant.
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
KmnO4 is a powerful oxidant, it probably burned your alcohol to give several by product.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
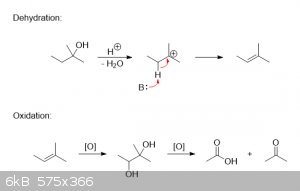
Was the KMnO4 solution acidic by any chance? Because contrary to popular belief, tertiary alcohols actually can be oxidized, at
least under protic conditions. While it's true that tertiary alcohols themselves won't undergo direct oxidation, they will, however, readily dehydrate
to an alkene in the presence of a strong proton donor. That alkene can then be oxidized to a vicinal diol, and then further to a ketone and/or
carboxylic acid.
In the case of 2-methyl-2-butanol, it would look something like this:
|
|
|
tshirtdr1
Harmless

Posts: 31
Registered: 12-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  |
they will, however, readily dehydrate to an alkene in the presence of a strong proton donor. That alkene can then be oxidized to a vicinal diol, and
then further to a ketone and/or carboxylic acid.
|
Yes, that's right. Tertiary alcohols readily undergo elimination to yield alkenes, then the KMNO4 reacts with the alkene to give syn dihydroxylation.
|
|
|
Stibnut
Harmless

Posts: 43
Registered: 4-6-2015
Member Is Offline
Mood: No Mood
|
|
Good call! The KMnO4 solution was pH 5.2, according to my $13 Chinese-made pH meter. I just put in some NaOH and brought it up to 8.0, then tried the
reaction again using acetone as the solvent. No color change and it's been about 15 minutes. I was about to send a question to the vendor.
Another question: even acetone by itself, using the old acidic solution, will cause the color change after 1-2 hours. My acetone is denatured with
denatonium benzoate, but (supposedly) has no other ingredients. Is the change because of the denatonium, or would it be because of other impurities in
the acetone, or will acetone itself experience something similar to the tertiary alcohol in acidic conditions, or will acetone slowly oxidize to
something anyway in KMnO4?
If it's the denatonium or something else, does anyone have any opinions of Klean-Strip acetone? I'm thinking I'll replace my current now-empty bottle
with that stuff, which doesn't appear to list denatonium or any other ingredients besides acetone.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Enolizable ketones like acetone are slowly oxidized by KMnO4. Addition of acid or base promotes enol formation, which is a necessary intermediate here
and probably greatly accelerates reaction.
Kleanstrip everything is fine. I've used most of it for various things. It evaporates with no residue.
|
|
|