| Pages:
1
2
3
4 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have made dry chlorine probably about 10 times so will make some comments that may be useful:
1. I dried my Cl2 with a fritted bubbler submerged about 2" in con H2SO4. CaCl2 prills may also be adequate but I can't quantify that, and I don't
know what degree of dryness you require.
2. As shown you have no system backpressure but if any is generated it may push Cl2 up through your stopcock (when open) leading Cl2 to the
atmosphere. The solution to this is a p-e funnel.
3. As you have no suckback liability the first empty tube likely is not needed. I used one also but I now think it was never needed. The Cl2 is
always ≥ atmospheric pressure.
4. PVC is quite satisfactory but will be degraded if the Cl2 is wet, which it is before the dryer. I used PVC as much as possible and considered it
sacrificial.
5. I made shrink fits of the PVC to 5mm glass tubing by heating the PVC end in boiling water before pushing it onto the glass. These very tight
connections never leaked.
6. I used 15%HCl dripped on dry TCCA per Len's method in Prepublication. This works well.
1 mole TCCA + 3HCl --> 3Cl2 + 1 mole isocyanuric acid
Have fun! 
[Edited on 15-5-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I would like a pressure equalizing drip funnel,
and there is definitely the potential hazard of HCl being ejected by back pressure,
this was the best I could come up with, without new glassware,
these are the kind of warnings that I am hoping for... thanks
The generator is too large, potentially 70 litres of Cl2 from 260 ml 36% HCl ... chemical warfare !
Is there a reason why I should not use 1M HCl or even less ?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Chlorine tend to bugger lots of things up, but not fast enough to be a problem if you consider things like corks, plastic tubing etc as disposable.
In the sketch it looks like the second test-tube should be facing the same way as the first one, and have the connector tube swapped if you wanted to
protect against suck-back.
A Magpie says, the chlorine pressure prevents suck-back, and that has not been an issue the few times i've tried it this way.
A non p-e addition funnel works fine, so long as you remember to keep an eye on it and keep it topped up.
Try to add the HCl drop-wise rather than getting all excited and dump a whole load in all at once !
One of the great things about Len's Cl2 method is that the addition of HCl controls the amount of chlorine produced, so if it's too much,
just stop adding HCl.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yes. Yields will likely be lower.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
15%HCl is about 4.4M according to my handbook. Len probably selected this as a practical concentration to use.
If you go down to 1M the reaction may be too sluggish. You could experiment using a test tube or small beaker.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
There's also the very important thing about Flask Volume.
If the acid is very weak, you'll quickly fill up the generator flask, mostly with water !
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sulamain's Dragon Gold
I've decided to go ahead and copy Sulaiman's experiment.
Naturally, as an amateur, things are never so simple as 'pick up a vial of powdered gold' etc.
I have a some Gold and a rusty old file.

Iron would definitely be a contaminant, so i can't just use that old file to get gold filings.
Dunk the file in agricultural-grade phosphoric acid for 24 hrs is the answer !

Pretty gunky after even 30 mins.

After 24 hours and some washing and brushing with a steel brush, all nice and clean.
(well, as clean as i'm going to be bothered getting it)

Finally, with a Clean(ish) file i can grab the gold between a pair of pliers and file it to get smaller particles.

Ironically the file is not that old, just that quite a lot of Chlorine gas got to it the last time i did a Chlorination !
OK Sulaiman, i got 0.79g of Au all broken down and ready for chlorination.
Over to you for the next step.
Edit:
Reading what i've written, it seems silly to Not dunk it in conc HCl overnight then rinse out any remaining iron contamination.
[Edited on 16-5-2016 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Adding some HCl was worth doing :

Funny, but i thought it'd go green due to the traces of iron, going yellowy later.
Unlikely that i've dissolved any Gold with just 20% HCl !
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
My adapter arrived yesterday, today I assembled the chlorine generator, pretty much as my earlier sketch.
No experiments yet as my prolapsed disk is giving me trouble, hopefuly soon .......
on the bright side, more time to plan a couple of Cl2 experiments.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Still hanging fire here with the Au still in the HCl.
Will wait until you're ready, seeing as it was your idea.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
please, don't wait for me, no way to know how long before I'm fit enough to continue.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Make benzyl chloride, a moderately effective tear gas and a great organic precursor chemical. Bubble chlorine into toluene (xylene would probably
work too, if you didn't care about further synthesis, or you actually want a mixture of 2-methyl, 3-methyl, and ortho and meta di-benzylchlorides).
Toluene can actually dissolve quite a lot of chlorine, and will eventually turn yellowish-green. When it looks like the chlorine bubbles aren't fully
dissolving in the toluene anymore, here's the fun part. Take a pinch of any bromide salt. Sodium bromide is available in small packets in pool
stores. Add it to the mixture, and you'll see some brown stuff come off of the salt. That's bromine and bromine monochloride. If you don't notice
anything else happening after a minute or two, gradually move it to brighter and brighter light sources. It should start bubbling like crazy with HCl
fumes, and the green will totally disappear.
That was a free-radical-initiated chain reaction. Bromine absorbs longer wavelengths of light than chlorine does, and thus a little bit of energy
from light will turn it into a free radical, where it will quickly grab a loosely-attached hydrogen atom from a nearby toluene molecule. That makes
toluene a radical too, which will grab any chlorine or bromine that happens to be nearby, forming yet another radical. This chain reaction will keep
going until the radicals run into each other and form a stable molecule.
You'll also notice a new smell. Well, not so much a smell as an attack on your senses. Benzyl chloride, when it comes in contact with water, forms
an equilibrium between itself and benzyl alcohol plus HCl. Much fun when it contacts your mucous membranes. Anyway, if you manage to isolate a
significant amount of it via distillation, you can convert it to benzyl alcohol by stirring or shaking with an alkali hydroxide solution. Benzyl
alcohol smells faintly pleasant, but you've probably got enough benzyl chloride around to overpower it. (seriously, that stuff will adsorb onto
virtually any polymer and then slowly release over weeks). So if you want a more pleasant smell, crack open a "Heavy Duty" carbon zinc battery,
preferably one of those 6-volt lantern cells. The outsides are made out of zinc (useful for lots of stuff), there's a graphite rod in the center of
each cell, (great for electrolysis when you don't want metal dissolving in your solution), and packed around each rod is the bulk of the battery in
black powdery form: manganese dioxide. Take some of that stuff and mix it with your benzyl alcohol. You'll notice a nice floral smell, like vanilla
or cherries. That's benzaldehyde. Congratulations, you've just made a DEA List I precursor! You've taken the first step towards becoming Walter
White. Expect a knock on your door from the DEA any day now.
[Edited on 6/1/16 by Melgar]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Dragons Blood ... Started
Feeling a bit better today, so trying first run of anhydrous AuCl3 synthesis.
Gold powder in 250 ml rbf, sand in solder pot, temperatures stabilised.

Chlorine generator and CaCl2 drier flushed with Cl2, 250 ml rbf flushed with Cl2, heated for a while, flushed, 30
minutes in ...
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
part2
couldn't add photo, 30 minutes in...

tbc
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
cool !
Glad you're feeling better.
[Edited on 4-6-2016 by aga]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
continued
After about 1 hour at 160 C;
chlorine fainter than 100 %
gold looks brown and the mobile dust has clumped
recharged with Cl2
temperature increased

my phone camera shows weak colours, the chlorine is quite yellow, the gold is a disappointing dark brown, and a reddish tinge on the inside of the rbf
can be seen
... to be continued ... as long as it takes ....
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Lob a small stirbar in there so you can fiddle with the gold/powder.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I don't have stir bars,
there is a hole in the rubber bung to allow for Cl2 consumption,
so far I've resisted 'poking' the gold, going now to check progress ...
EDIT 18:10 with some tapping and bumping the mass is now mobile/powdery again.
Flushed with fresh Cl2 , temperature increased ....

19:10 not much change but it appears as though gold + chlorine makes a volatile brown/red substance that sublimes onto the cooler part of the rbf,
possibly related to AuCl3 disproportionation ... looks good
... getting a little impatient, 19:20 ... heating bath at 252C ...
[Edited on 4-6-2016 by Sulaiman]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Well, you got gold(I) chloride boiling at 298 C and gold (III) chloride boiling at 254 C...
You're up at 252, which is pretty much close enough to be boiling away some of your product through the hole in the bung.
I'd drop it by 20 C or so, maybe add a condenser if possible : i.e. just a longer pipe on the flask.
More Edit:
wiki says :
"Anhydrous AuCl3 begins to decompose to AuCl at around 160 °C; however, this in turn undergoes disproportionation at higher temperatures to give gold
metal and AuCl3"
So drop way back down in temperature !
(or maybe go higher: wiki doesn't say what 'higher temperatures' means.)
[Edited on 4-6-2016 by aga]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
On the assumption that the (sublimed, disproportionated ?) substance that collects on the inner wall of the rbf is 'product'
I am willing to risk a little loss in return for reaction rate.
dry chlorine seems to react very slowly with gold
(like the dry/wet nail rusting experiment ?)
I'm going to risk leaving it overnight at its present 232 C
this is the sand (now sand + NaCl as sand spilled) temperature,
I do not know what the gold temperature is.
Some sort of powder/gas mixing arrangement would help.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Great stuff !
Experimentation as it happens !
I will definitely follow up and repeat your experiment, just as soon as i get a new, bigger extractor fan.
I'll try to do it real-time as well.
I think what you have done, real-time-wise, is a 'first' here on SM.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Last night I left my AuCl3 reaction vessel with a fresh charge of chlorine gas in the sand bath at 232 C.
24 hours later there is very little yellow tinge from the chlorine,
the gold powder looks like gold powder again, an there is a small quantity of red deposit on the flask wall.
Here is the gold powder still in the rbf (originally 0.58g)
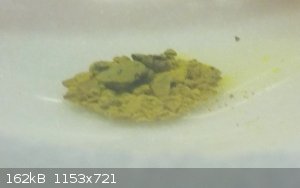
and after a recharge with chlorine, a view of the red stuff

Notice the gold has very quickly reacted ... on the surface only.
I hope that the red is not just decomposed rubber stopper 
tbc ....
Aside;
Due to paranoia I used too much silicone grease on the ground glass joints of my chlorine gas generator
... chlorinated dribbles of silicone grease are time consuming to remove 
[Edited on 5-6-2016 by Sulaiman]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Paranoia with Chlorine is a Good Thing.
Quite often i opt to Not grease the joints simply because the reagents will eat them and contaminate the product, and being a purist ....
... or more honestly, the vaseline just dribbles into the pot, so better off without it.
IF the red condensate is actually the Product, how you gonna get it out ?
[Edited on 5-6-2016 by aga]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I hope to dissolve the AuCl3 in diethyl ether and crystalise it from there.
If I had anhydrous ethanol I'd probably use that instead.
I've only once recrystalised from a solvent other than water, sulphur from DCM, and that was very small crystals,
I will try to evaporate slowly enough to form a visible crystal 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ether ! Eeeek !
Am i being a bit over-concerned, or is Ether not much of a bad-ass ?
|
|
|
| Pages:
1
2
3
4 |