aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Dean-Stark trap ?
This brand-new £7.28 inc shipping item looks pretty dean-starky to me :
http://www.ebay.co.uk/itm/381569370564?_trksid=p2057872.m274...

|
|
|
Eosin Y
Banned troll
 
Posts: 83
Registered: 8-5-2016
Location: Eton College science department
Member Is Offline
Mood: Aga needs to cool his heels
|
|
What would you usually use a Dean-Stark for?
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Looks very Dean-Starky, aga. I might even grab one.
@Eosin Y, https://en.wikipedia.org/wiki/Dean-Stark_apparatus
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Eosin Y
Banned troll
 
Posts: 83
Registered: 8-5-2016
Location: Eton College science department
Member Is Offline
Mood: Aga needs to cool his heels
|
|
Thanks. So this would be useful for something like separating ethylene glycol from water in antifreeze or something? I might grab one too, as it might
be useful and it's a low price.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It might need tipping to the side a bit to work.
I have a 'proper' one (but without the stop-cock) and the arm is slightly angled downwards.
For that price it's a steal !
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Score!
Got me one.
1. Purifying immiscible substances that have an azeotrope with water. Toluene is an example. Barely soluble in water but has an azeotrope.
2. Dehydrating substances that are temperature sensitive. Oxalic acid dihydrate is an example. You can't just heat it to drive the water off. But
if you dissolve it in toluene you can drive off the water using the toluene water azeotrope and return the toluene back to the original flask. Then
you have a dry solution of the oxalic acid which you can then use to form the anhydrate.
3. Essential oil distillation -- which is what this one is advertised for. You could I guess use a sep funnel but the volumes you are working with
make it not really workable. The Dean Stark does a better job and does it continuously as you perform your steam distillation.
Probably other uses as well. I have never used a DS trap but have been intrigued by the process. There have recently been a couple of good videos on
it from Nurdrage, NileRed and I think also Doug's Lab as well. At this price, how could you turn it down?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
For US eBay:
http://www.ebay.com/itm/24-29-50ml-Glass-Oil-Water-Receiver-...
Cheaper even than the UK listing.
[Edited on 12-5-2016 by careysub]
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eosin Y  | | Thanks. So this would be useful for something like separating ethylene glycol from water in antifreeze or something? I might grab one too, as it might
be useful and it's a low price. |
You won't need DS to separate EG from Water. A simple column will do the job as the BP of EG is 197 Deg C.
Amongst its several uses, I use it to remove reaction water from immiscible lighter solvent which is refluxed back to the rection flask.
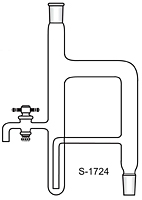
BTW there is a modified version of DS which can be used for solvents heavier than water. IIRC it is described in Vogel.
gsd
This image is from the net not from Vogel
[Edited on 12-5-2016 by gsd]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
I'd rather spend the extra bit to get a proper graduated one. You can't work blind as I say, good steal if you do not need to track any results.
Good grab for those who like toys though for sure, great find.
http://www.ebay.ca/itm/15ml-24-40-Glass-Distillation-Receive...
One I plan to get.
[Edited on 12-5-2016 by XeonTheMGPony]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That is a comically low price for any glassware, it is amazing how cheap things are in China, plus they subsidize the postage rates for exported
goods, so it costs almost nothing to mail things out of China.
I can't blame anyone for buying things that cheap, but if anyone in the US wants a Dean Stark, I have several in 29/42 size, which I would sell
cheaper than for a 24/40, since they are so uncommon a size. I can even thow in an adapter. It is not worth selling them to Europe, Asia or Aust,
from here, as the US has raised postage so much in the last couple years that shipping anything out of the US is way too expensive. Does that maybe
help explain the trade imbalance with China? I can buy items from China with shipping for much less than just the postage cost to ship the same
size/weight package back to China. That is just wrong.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dr.Bob  | That is a comically low price for any glassware, it is amazing how cheap things are in China, plus they subsidize the postage rates for exported
goods, so it costs almost nothing to mail things out of China.
I can't blame anyone for buying things that cheap, but if anyone in the US wants a Dean Stark, I have several in 29/42 size, which I would sell
cheaper than for a 24/40, since they are so uncommon a size. I can even thow in an adapter. It is not worth selling them to Europe, Asia or Aust,
from here, as the US has raised postage so much in the last couple years that shipping anything out of the US is way too expensive. Does that maybe
help explain the trade imbalance with China? I can buy items from China with shipping for much less than just the postage cost to ship the same
size/weight package back to China. That is just wrong. |
I'd love to get stuff from USA suppliers but the shipping is akin to rape! For a 5 dollar part some are quoting 30 + dollars for shipping! when that
part is the size of a potato chip!
I just can not justify that!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Regarding the use of a Dean-Stark trap, I have one and I've only used it once, but it was quite helpful when I did need it. I used it when I was
making p-toluenesulfonic acid. As mentioned upthread, toluene and water are immiscible but form an azeotrope. In the reaction between toluene and
sulfuric acid, p-toluenesulfonic acid and water are formed, so the reaction is driven forward if water is removed. With the Dean-Stark trap, it is
very easy to remove water while the toluene that is collected flows back to the reaction flask. Very nice to just sit back and watch it go.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
They are also quite useful for soxhlet extractions of wet material.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What does that setup look like ?
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Something like this:
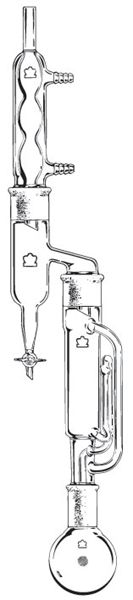
We're not banging rocks together here. We know how to put a man back together.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
The Dean-Stark + Soxhlet arrangement would require a very specialized Dean-Stark trap, or (more likely) a similarly specialized adapter since the
upper joint on Soxhlet extractors are huge and often odd sizes (40/38, 50/42, 34/45, 45/50, 55/50, etc).
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Interesting! I want to try that now. I have a small 34/45 Soxhlet and a 34/45 to 24/40 adapter, so I could actually set that up.
What would be a specific example of something that you would use that apparatus for?
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I've used it a couple of times for toluene-extraction of wet (moist) material. Useful if you're worried about loosing volatile components or simply
want to dispense with a separate drying process. It was a purpose-made adapter, not dissimilar to the one depicted.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
I ordered one of these from playtle on eBay (only $10.24 shipping included) but it was shipped in semi-soft packaging that is manifestly inadequate to
protect that very fragile side arm, and it arrived broken off. I cannot imagine these things ever get through unbroken the way it is being packaged.
I am currently corresponding with the seller about addressing this.
Just thought I would give a heads up if others want to order this. I will post how this comes out.
It is cheap enough for write-off so it is not a big deal.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
If anyone really wants to do this well, I do have one 45/50 Dean-Stark that I remembered when I saw the photo above, I also do have a few Soxhlet
apparati as well, in several sizes. It has taken me a while to find matching tops and bottom sets, as they make them in about 5 or 6 various joint
sizes on the condenser bottom. The 45/50 Dean Stark holds at least 100 ml, so using it only makes sense with a pretty large bottom flask and a large
scale extraction. I have a few other 45/50 joints adapters as well, gas inlets, claisen, dist head (I think), and some other large stuff, but just
one box of it. Not sure how much demand there is for that size glassware.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
the other half
the glassware poined to above
has a sibling http://www.ebay.co.uk/itm/24-40-Glass-Oil-Water-Receiver-Sep...
one to remove the less dense liquid, one to remove the more dense liquid.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Mine arrived today.
Not sure it was supposed to arrive as a kit of parts 

|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Exact same condition as mine.
The way they are packing these things they CANNOT arrive unbroken!
The mailer their are using is a Tyvek envelope containing one of these:
http://www.alibaba.com/product-detail/Hot-china-products-who...
The air tube are rigid along their length, but they flex rather easily side to side, and thus the side arm WILL get broken off.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | but they flex rather easily side to side |
Yep. Seems to be exactly because of that.
It's a real shame as the Quality of the glass & ground-glass valve all seems pretty high.
A couple of pieces of cardboard could have saved it.
[Edited on 2-6-2016 by aga]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
The graduations are the wrong way round. They start at 0 part way up, not even the level of run off, and go up as you go down.
|
|
|