| Pages:
1
2
3 |
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Fluorine for Laser
If I get 5 grams of copper (II) fluoride and heat it to 950C (which produces fluorine gas), how much of this gas do I get?
Also, I have vacuum equipment, (through UHV) will placing this in vacuum to lower boiling point also lower the temperature it would need to
release the fluorine?
DON'T THINK YOU KNOW MORE ABOUT SAFETY THAN I DO. I can program servomotors to remotely actuate this if I must.
Argon Fluoride lasers are used in 193nm immersion lithography
I understand it is difficult to contain fluorine once at such a temperature.
[Edited on 22-6-2016 by SupaVillain]
[Edited on 22-6-2016 by SupaVillain]
Oh.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Quote: Originally posted by SupaVillain  | | If I get 5 grams of copper (II) fluoride and heat it to 950C (which produces fluorine gas), how much of this gas do I get? |
I find it hard to believe that it decomposes to fluorine gas. In fact, I'm certain it doesn't. What's your source?
Quote: Originally posted by SupaVillain  | | Also, I have vacuum equipment, (through UHV) will placing this in vacuum to lower boiling point also lower the temperature it would need to release
the fluorine? |
You will damage your very expensive UHV setup.
Quote: Originally posted by SupaVillain  | DON'T THINK YOU KNOW MORE ABOUT SAFETY THAN I DO. I can program servomotors to remotely actuate this if I must.
Argon Fluoride lasers are used in 193nm immersion lithography |
I get that it bothers you when people tell you not to do stuff because it's not safe. I still recommend you listen to what others have to say about
safety though. Taking advice from others is basic safety.
If you really want to risk your life with fluorine gas, you might be able to buy it 10% diluted in argon (they sell it where I live). I wouldn't
suggest it though. Can't you make a different laser?
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
I think the OP is referring to Cobalt(III) Fluoride? That does decompose I think, giving off F2 gas.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Although that sounds vaguely familiar, this paper says that thermal decomposition of salts cannot yield significant fluorine because the temperatures
required would cause the fluorine to react with any reasonable reactor material. It does propose an alternative chemical means of preparing fluorine
at more significant yield, but looks complicated.
http://pubs.acs.org/doi/abs/10.1021/ic00241a001
Edit: ok so manganese(IV) fluoride does decompose to fluorine in that source above, that's probably the salt you were thinking of
[Edited on 22-6-2016 by DoctorOfPhilosophy]
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I find it hard to believe that it decomposes to fluorine gas. In fact, I'm certain it doesn't. What's your source?
|
http://www.sciencemadness.org/talk/viewthread.php?tid=16246&...
the fourth post on this second page of the above link
| Quote: |
You will damage your very expensive UHV setup.
|
I can use my pumps to take a plumbed glass pipe or bottle down to 10^-4, after putting the copper fluoride inside. Argon can be leaked in and fluorine
can then be evaporated. An induction coil can bring heat to be more concentrated upon the copper compound, rather than using a flame. .....Actually a
smaller plumbed glass container could hold the copper fluorine and then be closed off and disconnected, leaving only desired vacuum and gases inside a
tube.
| Quote: |
Taking advice from others is basic safety.
|
Yes, but you see I did not come here until I had done hours of research over the whole topic, and seen videos of others handling the chemical, and
surviving their activities. Such is the proper way rather than only being educated through the warnings of an online forum. I was astonished to see
this individual in such a lack of PPE with fluorine. https://www.youtube.com/watch?v=M5_9z1TxUfg
There are large projects I have longed to complete but have not, due simply to a temporary lack of adequate safety measures.
[Edited on 22-6-2016 by SupaVillain]
Oh.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
To quote from the thread you linked to
| Quote: |
To preface: I am a fluorine chemist with ~8 years of handling HF, F2, Metal fluorides, XeF2, etc etc etc etc.
There is so much inaccurate information in this thread that I'm not even going to start to address, instead I'll focus on the posters question.
CuF2 --950C--> Cu + F2
While on paper this may be possible, feasibly it is not. You will not be able to find a reactor that is stable to fluorine at these temperatures.
Graphite will react. Teflon will degrade. Even corrosive resistant metals and alloys such nickle 201, inconel, monel, etc will react and flake away.
HF/F2 is dangerous...but it can be handled safely. Oddly enough, in my corresponences with docters who treat HF burns, the group of people who most
frequently are admitted and treated for HF burns are people who have fancy expensive rims on their cars. The cleaners for these aluminum rims has HF
on it and they don't read the warning label and don't wear gloves.
|
I guess your question is, does doing the reaction in a vacuum lower the temperature enough to make is feasible? My educated guess is no, but if you're
going to test it, be careful and good luck!
The guy in the video is not wearing a mask, but even if he did I'm not sure it would help him much against fluorine. I've caught a whiff of HF 50% and
it wasn't pleasant, but I haven't suffered any obvious harm yet, as far as I can tell.
[Edited on 22-6-2016 by DoctorOfPhilosophy]
[Edited on 22-6-2016 by DoctorOfPhilosophy]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
CuF2 certainly will not decompose to Cu and F2 at 950 C. Maybe, just maybe, you can get it to decompose to CuF and F2 if you throw enough heat at it.
CoF3 indeed can be used to make elemental fluorine. It decomposes to CoF2 and F2. It requires a temperature of 600 C or so.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Well the manganese (IV) fluoride might be ideal because it makes a nonreactive powder but it is not available anywhere that I know of. CoF3 it is
then. Only problem is that I will need to keep it desiccated and dry to avoid generating HF. I need the tiniest little pinch that makes all the
difference apparently. "Gas mixture: 2.5 t F2, 75 t Ar, 2300 t Ne".
https://www.cymer.com/files/pdfs/Technology/1991/Argon_Fluor...
http://www.rit.edu/~w-lith/research/immersion/SPIE_5040_58_i... (diagram)
Thank you for your help. These links might help others looking for the same thing via search engines.
Oh.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You have equipment that can handle F2 at 6000C but you don't know that the substance you talk about does not liberate
F2.... Still you mention ''DON'T THINK YOU KNOW MORE ABOUT SAFETY THAN I DO. ''.
Good that you CAPS LOCKED it, otherwise I probably wouldn't have understand.
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Be sure to report back your results, you would be the first forum member to make elemental fluorine as far as I can tell, and that is a pretty good
accomplishment.
Also, please do not forget to include a writeup of your method for making Co(III)fluoride without using elemental fluorine.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
CoF3 is a nasty compound. I have 10 grams of it and it is extremely reactive and only can be stored in an ampoule for extended periods of time. The
inside of the ampoule is etched and becomes opaque but the ampoule survives.
|
|
|
ParadoxChem126
Hazard to Others
  
Posts: 104
Registered: 5-4-2013
Location: USA
Member Is Offline
Mood: No Mood
|
|
Luckily for the OP cobalt fluoride appears to be available on eBay for the mere price of $70 per 25g 
This probably isn't going to work. Assuming you manage to produce some amount of fluorine gas, you probably won't be able to build a useable laser
from it. You will have to build everything from fused quartz, as normal glass absorbs deep UV (the laser operates at 193 nm). Also, I'm fairly certain
that fluorine will react with normal glass anyway, let alone at high temperatures. Using a torch and heating the glass itself is unavoidable because
you eventually have to melt and seal the optical cavity.
Assuming again that you have the facilities and experience to successfully carry out quartz glassblowing (i.e. high temperature torches, annealing
oven, etc.), you probably won't be able to create an atmosphere which will function properly as a laser. I'm not an expert with these argon fluoride
lasers, but a quick search on the web shows that the operating gases are contained at high pressures within the cavity. Thus, you need to devise a way
to compress your argon-fluorine mixture from a vacuum to high pressure. Also, the ratio of the gases is another important parameter in other gas laser
systems, so I assume it holds true for argon fluoride lasers as well. You need to find a way to have precise mixture control at very high pressures,
which is pretty much not feasible in an amateur setting.
Amateur laser building is no simple feat. I recommend you first build another easier laser, perhaps a helium neon laser. Honestly, the actual
production of fluorine seems like a miniscule task in the grand scheme of your laser plans.
You will also run into problems trying to induction-heat solid CoF3. It is not electrically conductive until it is molten, so eddy currents cannot be
induced. I wouldn't risk your expensive vacuum equipment, and it is not likely you would have much success with this unless you have the
aforementioned abilities and materials.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by woelen  | CuF2 certainly will not decompose to Cu and F2 at 950 C. Maybe, just maybe, you can get it to decompose to CuF and F2 if you throw enough heat at it.
CoF3 indeed can be used to make elemental fluorine. It decomposes to CoF2 and F2. It requires a temperature of 600 C or so. |
Hmm. This raises the possibility in my mind of making a fluorine element ampoule by sealing a small amount of CoF3 in a quartz tube under vacuum, then
heating to 600 C.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Would it not react again as soon as you stopped heating to 600C?
On the feasibility of building such a device in general
SupaVillain, your excimer laser project intrigues me so I did some reading. Looks like the laser cavity can be built of a stainless steel tube with calcium fluoride windows, which are only $90 per piece. Electrodes run the length of the tube, so you'll need to suspend them on insulators. This source says they use metal and ceramic for the construction, probably Al2O3 insulators, such as these (see patent below).
The excimer laser needs high pressure, but not too bad, about 6 bar absolute (source). It says here 99.9% of the gas is neon buffer, and seals are metal, but it seems suspicious.
This source sounds more realistic:
F2 (0.2%)
Rare noble gas (2%)
Neon buffer (balance)
According to this patent, tin coated Inconel®-718 is a good material for seals, but Viton seals are OK too. About the electrical feedthroughs:
| Quote: |
Cathode 18 and each of the 15 feedthrough conductors carrying peak voltages in the range of 16 kv to 30 kv must be insulated from the metal surfaces
of enclosure 10 which is at ground potential. Because of the corrosive F2 environment inside the chamber only certain high purity ceramic insulators
such as high purity A2lO3 [sic] can be used for the portion of the feedthrough assemblies exposed to the gas environment.
|
MDC sells alumina HV feedthroughs.
About your original question though, just buy excimer laser gas. It won't be cheap but at least you eliminate a major variable from an already extreme engineering challenge. Maybe you
can get some sponsors or something?
On obtaining the fluorine
Ok so I have a new idea for your laser. Attach a sidearm to the laser cavity with a heated boat containing CoF3. Fill laser with other gasses to
required pressure and seal the chamber. Heat the CoF3 and equilibrium shifts toward F2. At the desired percentage of F2 (ie. when the laser starts
working), stop increasing the temperature. To service the cavity, cool the sidearm, equilibrium shifts toward CoF3. Once it's totally cold it should
be more safe to open the tube.
Assuming we need F2 at 0.2% and 6 bar, that's a partial pressure of 12 mbar. The laser cavity has to be big, for reasons discussed below, so let's say
1 litre. Ideal gas law says ~0.5 mmol of F2 (http://www.wolframalpha.com/input/?i=ideal+gas+law+12+mbar+2...), so if our equilibrium is 2CoF3 <=> 2CoF2 + F2, we need about 1 mmol of
CoF3 (116 mg) to convert to fluorine. If you buy 25g of CoF3 on eBay, then only 0.464% needs to convert to F2. Since your target concentration is only
0.2%, this does seem plausible.
The reason the cavity has to be big is because after each discharge, the laser gas needs to recover in the dead space of the cavity. In the commercial
laser they use a cage blower along the whole length of the cavity to keep the gas flowing past the spark gap. A simpler solution is to use convection
at the expense of lowered pulse repetition rates. Heat the bottom of the cavity and cool the top to create a flow. The recovery time can be on the
order of 10^-1 seconds so ignoring this requirement and building a very narrow tube just big enough for the electrodes will give you very poor pulse
rates.
The main challenge with this method is that a lot of fluorine will be consumed after initial startup due to the internal components being passivated.
That will leave you with a lot of CoF2 which is not good for your equilibrium. A possible solution is to make a larger side arm of the same design to
generate fluorine for initial passivation. Heat the passivation sidearm as high as possible and after some time, cool down to consume excess F2. Then
seal off the sidearm from the system entirely to prevent remaining CoF2 from consuming F2 produced later.
[Edited on 22-6-2016 by DoctorOfPhilosophy]
[Edited on 22-6-2016 by DoctorOfPhilosophy]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
My guess is partially, depending on the system equilibrium. To put it another way, how efficient a fluorine scavenger is CoF2 in a CoF3/CoF2 mixture?
I expect to form CoF3, a substantial partial pressure of F2 and an excess would be used. In this case recombination is occurring with a stoichiometric
ratio, and a residual very low F2 partial pressure.
Also - apparently you can cool quartz tubes from 600 C to room temperature by dropping them into water without having them crack. Such rapid cooling
would tend to suppress recombination.
[Edited on 22-6-2016 by careysub]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I worked in a fluorine chemical company for a few years, long ago. So there is a member who worked with fluorine and lived to tell, but that was
with the correct equipment. We used CoF2, which was heated with fluorine to generate CoF3 inside a Monel reactor, then other materials were passed
through the CoF3 to fluorinate them. So yes, that is a source of F2, but not a trivial one, as every leak generates a fire or toxic event. The
waste HF, F2 and all other effluent was also put through a large acid scrubber, so that we would not pollute the air or kill our neighbors. They do
seem to appreciate that.
Doing F2 chemistry without the proper equipment and materials will be tough, it corrodes many things, and vacuum pumps are likely one of them. If you
can get the F2 in argon mixture made up, it will almost certainly work much better. But you can always try it, just read some older books on the
subject if you can, they are from the era when people had to cob together things and use crazy ways to do things.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
For Fluorine Generation:
I did not expect to get so many replies, I honestly thought this thread was over and only popped back in to add some other fluorine generator info
that I accidentally found in the excimer laser googling I was doing.
Larned B. Asprey's Asprey salt...above 300C according to bottom-most link.... 2[K2NiF6 * KF](s) = 2K3NiF6(s) + F2(g)
(info here - https://books.google.com/books?id=2OJRAwAAQBAJ&pg=PA424&...)
and here applied to lasers
aries.ucsd.edu/LMI/TUTORIALS/excimer-primer.pdf
___________________________________________________
For the excimer laser application
Thank you for the support in calculation and in informing me that high pressures are reached. Perhaps the lasing glass and electrical feedthroughs
could be made of sodium silicate/water glass. I did a bunch of research a while back in finding that it is near zero outgassing, dielectric(at least
as much as plate glass), and very strong when cured. I was aware of the calcium fluoride "windows" being expensive, as mtixtl.com sells them for about
$79USD a square centimeter....Hold on, the above thorlabs link has a sq. inch for $115, and if it could be cleaved into 4 separate sq. cm's, this
might suit however many it needs. I still need to review this laser schematic, to determine all the interior optics needed However, if costs can be
minimized elsewhere, then this won't be a problem. I am interested in figuring out how to make this as I am finishing a SEM. It is the electronics,
mostly that is taking me forever to complete it. I was under the impression that E-beam lithography could be used for chip-fab but adequate
performance is now only obtained via 193nm immersion lithography.
Oh.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Huh, that's a very cool salt. Looks like they basically had the same idea as me, having an integrated module of salt connected to the laser cavity.
Getting potassium hexafluoronickelate(IV) might be a pain, but not impossible. Looks like it's around $90 for 10 grams. Naturally, you need fluorine
gas to make the salt so it would be pointless to do that.
If you get it working I'll send you some silicon wafers to kickstart your fab. I tried making mosfets before with limited success but I've moved on to
microfluidics because there are more people interested in working together on that. What photoresist are you planning on using for this? That stuff is
like $500 per bottle and the smallest spec of dust ruins it!
PS. Crunching the math again for potassium hexafluoronickelate(IV) suggests that you will need at least 0.25g per litre. Not too bad...
[Edited on 22-6-2016 by DoctorOfPhilosophy]
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
That's very kind of you, thanks for the offer. I'm not sure which photoresist I would use but am looking to replicate graphics processing units and
such because of their ability to work for artificial neural networks. I guess I would use whatever is used by the manufacturer of the specific
graphics card. The new kilocore is 32nm and after looking at its publication I'd like to replicate it... but I would have to make merely a similar one
based off info in the paper as I won't have one in my hands to work with.
Microfluidics is pretty interesting. I've looked into DNA oligonucleotide synthesis in a PDMS lab on a chip for about 300 to 400 USD IIRC
I think I'm still sticking to CoF3 generation of fluorine.
Oh.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I'm not an expert at L.A.S.E.R. technologies, so sorry if the ideas are dumb...just ideas...
CO2 lasers do exists...could it be possible to use CF4 instead to circumvent the use of dangerous F2?
What about SiO2 (Al2O3/Cr2O3 laser do exist) or SiF4?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
any ideas are welcome, unfortunately it is the argon and fluorine combo that makes it at the 193 nm wavelength, which is what is required for the
application. Solid state lasers don't have much written about them and don't seem to generate enough power like gas lasers.
Also, this paper:
193-nm lithography at MIT Lincoln Lab.
Cover Art image
by Michael Hibbs, Roderick Kunz
Format:ArticlePublication year:1995|Peer-reviewedSource:
Solid State Technology Jul95, Vol. 38 Issue 7, p69
Editions statement:No other Editions or FormatsDatabase:Academic Search Complete
...seems to say that fused silica, SiO2, is good enough for the optics, and that CaF2 can be used as well for slightly less damage...
According to Hibbs and Kunz (1995) "Due to its crystalline nature, calcium fluoride (CaF2) does not suffer from compaction. It is susceptible to
color-center formation, but apparently this is caused only by defects and impurities in the material. High-purity CaF2, now available, is comparable
to or better than fused silica in its resistance to damage. At present, the main barriers to extensive use of calcium fluoride as a 193-nm lens
material are residual stress birefringence and lack of experience with the material's grinding and polishing characteristics.
Reticle-protecting pellicles made by Dupont and Exion have been tested at 193 nm for transparency and damage resistance. These pellicles, designed for
use at 248 urn, are made of proprietary Teflon-like materials that have very good transparency at 193 nm (absorption losses are in the range of
1--2%). Both brands were thinned by prolonged exposure to high-intensity 193-nm radiation and could eventually be ruptured. However, extrapolation
from the high-flux tests to the low-flux environment that the pellicles would actually experience in manufacturing indicates that pellicles would have
an acceptably long lifetime for manufacturing as long as photoresist dose requirements do not rise much above the 15 -25 mJ/cm2 range.
Except for concerns about pellicle thinning, materials used for binary masks at 248 nm are expected to exhibit substantially similar performance at
193 nm. Chrome opacity is as high at 193 nm as it is at 248 nm and optically induced damage to either chrome or fused silica is of little concern in
193-nm photomasks due to the low power-densities at the mask plane. The bulk absorption of fused silica used for photomasks does not have to be as low
as that used for the projection optics because of the much shorter optical path length through the mask. Considerable work is needed in the
development of phase-shifting materials for 193 nm"
Also according to Hibbs and Kunz (1995) "The projection optics of the 193-nm Micrascan prototype are patterned very closely after the 248-nm Micrascan
II optical design. The exposure field is a 22 x 5 mm slit through which the wafer is scanned along the short axis to give a total exposed area of 22 x
32.5 mm. The NA of the projection optics is 0.50 and the demagnification of the system is 4 x.
The optics consist of a concave mirror used as a 4 x reduction lens. A polarizing cube beam splitter sits between the mirror and the wafer surface.
Several refractive optical elements are included for aberration control. All refractive elements, including the cube beam splitter, are made of a
selected grade of fused silica. The cube beam splitter, which accounts for the greatest part of the optical path length in fused silica, was tested
extensively for transparency at 193 nm before fabrication. Its bulk absorption is <0.7% per cm. The optical transmission of the 193-nm projection
optics is approximately half that of the equivalent 248-nm optics, leading to a concern about lens-heating effects at 193 nm. At the low power levels
used for the initial lens-heating tests (about 32 mW/cm2 at the wafer plane), no definitive lens-heating effects could be seen. A more careful study
of these effects must be undertaken when higher laser power is available.
Scattered light is a serious concern at 193 nm. Since scattering from point-like defects (Rayleigh scattering) increases as the inverse fourth power
of the wavelength, the problem could be twice as pronounced at 193 nm as at 248 nm. Measurements have indicated that scattered light levels in the
193-nm optics are generally higher than in a similar 248-nm optical design. Future tests will have to establish whether the scattering is dominated by
defects in the bulk-fused silica, or by surface finish or coating defects. Specialized measurement equipment may have to be built for accurate
measurement of low-angle scattered light"
As for the construction of the laser, I think the best way is to simply understand that the fluorine will always affect whatever you
use over time, so a cheaper and disposable device is adequate. Therefore, I'd like to make the chamber out of copper pipe as according to NASA, (http://oce.jpl.nasa.gov/practices/2206.pdf) copper and nickel, or "monel" which is both, are what you should use for high pressures for fluorine.
Even brass would be fine for this. Remember that is for pure fluorine, whereas I'll be working with a tenth of a percent. Also, the
burst pressure for copper pipe is well over 93atm, whereas this will likely be at 3atm. As far as putting the gases in and getting to 3atm or more, a
simple application of Gay-Lussac's and Boyle's laws will do the work. I think the fluorine and argon will go in first, then a pipe full of neon, all
at atmospheric pressure, will be cooled with dry ice down until I can push a stopper in the tube forward to lower the desired volume, then close a
valve and wait for it to come back to room temperature, which would have it at its desired pressure if done correctly. That filter thing in the
patents is only for Krypton Fluoride lasers it seems, so I can eliminate that, then, whatever way I want to cool it, gets rid of that whole block, and
then the fan, well if i can put some magnets in some copper enclosure and have something whipe a magnet on the exterior, along a second connected
pipe, that would likely cause enough movement to be better than nothing, or i can attach some alumina strips to the same and do a magnetic stir bar
type of fan.
I have a little handheld bike tire filler that opens small cartridges of CO2 or Argon, (the argon will only be 3% of the mixture) but neon doesn't
seem to come in these cartridges. I may have to get a lecture bottle, and storing one is an issue, might have to get it and return it for each refill,
then again I'm not planning to produce 400 wafers an hour either.
[Edited on 24-6-2016 by SupaVillain]
Oh.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
You're right, silica will handle fluorine, take a look at how insanely dry it has to be and how difficult it is to prepare everything http://theodoregray.com/periodictable/Elements/009/index.htm...
The magnet idea might mess with the arc between the electrodes but I think convection should be good enough. For neon gas, take a look here (www.advancedspecialtygases.com/Neon.html). Otherwise, see if you can find a neon sign maker who will share some neon.
Here's a pic of my CO2 laser, and even that has taken months and an entire side project of building a sputter coating machine for the mirrors (the
whole setup on the right)
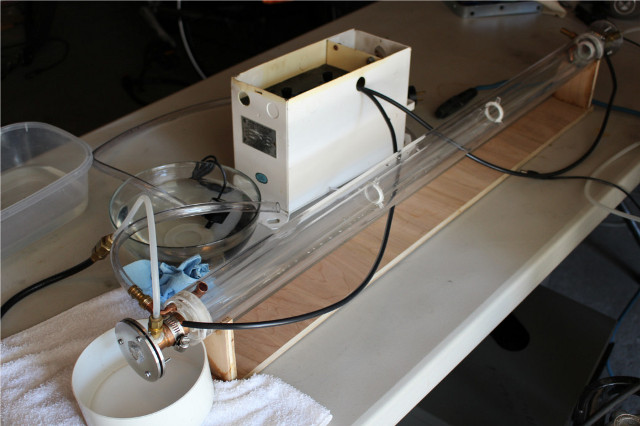  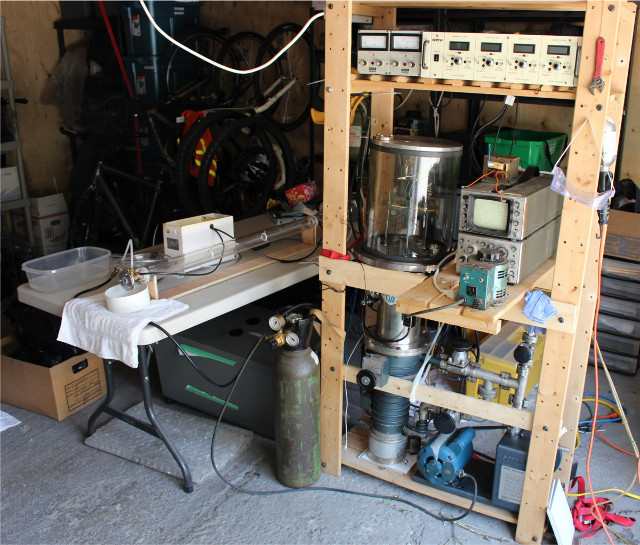
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Beautiful setup! Inspirational to say the least. Yes it's the side projects that hit me most with time. Especially learning electronics. Oh, and
waiting for eBay packages to arrive. Yea if advanced specialty gases can mail me their smallest bottle that would be great, I wonder how much that
would cost, there is one neon shop local to me I'll have to look into first. I already have some Viton gasket and some silver solder for the copper.
The patents have influenced me to take the interior fan more seriously.
The theodoregray link is very informative, I think the name of the DuPont fluorocarbon grease is "Krytox", I'll have to look into what to use to
"silylate" the quartz lenses.
Oh.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
I don't think i'm going to silylate the fused silica lenses, but definitely going to put krytox around the whole inside. Neon has been difficult to
find, called 5+ places today mostly sign stores that do neon signs but none of them, even the Airgas PLANT sell it. Found there's an Air Liquide in my
state and a Praxair just over the state border. Sent quote requests to both today but then I found this (https://www.nglantz.com/67786/Category/Neon-Gases). Might be a helpful link for anyone else interested. Used a calculator with the gay-lussac's
law and turns out I would need liquid nitrogen for my previous filling plan, then realized if I got a pressurized cylinder, I wouldn't have to do that
at all. The cylinders for 25 liters and below seem to be disposable and below 2 feet tall.
Also, wanted to share this, this source for fused silica optics is FAR cheaper than any others I've seen.
http://escooptics.com/products/windows-commercial-quality-sq...
Oh.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Maybe buy a plasma ball to get some rare gas neon, argon or xenon...but sometimes it is a mix of gases (I don't know if it is a problem for your laser
application)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
2
3 |