| Pages:
1
2
3
4
5 |
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Probably they were used as food additives, as all of them (sodium sulfate, bisulfate, metabisulfite, ammonium chloride) find an application as food
preservatives.
Does the box hint at anything else? I assume the compound names were the first 3 lines, since I could spot a Natriu (sodium) and Ammoni (ammonium).
Can you translate what the description says in the last 2 lines?
Also, a quick way to identify whether the first is sodium sulphate or bisulphate, is to simply add it to a solution of a carbonate. The bisulphate is
acid and will react with the carbonate to produce CO2. If you see bubbles, you have your answer.
[Edited on 3-8-2016 by Metallus]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | Probably they were used as food additives, as all of them (sodium sulfate, bisulfate, metabisulfite, ammonium chloride) find an application as food
preservatives.
Does the box hint at anything else? I assume the compound names were the first 3 lines, since I could spot a Natriu (sodium) and Ammoni (ammonium).
Can you translate what the description says in the last 2 lines?
Also, a quick way to identify whether the first is sodium sulphate or bisulphate, is to simply add it to a solution of a carbonate. The bisulphate is
acid and will react with the carbonate to produce CO2. If you see bubbles, you have your answer.
[Edited on 3-8-2016 by Metallus] |
Yes, those was the compound names, (see the translation, bold in last post. first one is more than sulfate I can read
...osulfate I am not that educated in Russian chemistry, so I am not sure what it starts with.
That piece was all that gave any info. Nothing more.. :/
Last two lines are company name "biochemreaktive" and like factory number or something..
One more problem is that I don’t know which bag is which compound..  I will
add carbonate to all of them and we will see.. I will
add carbonate to all of them and we will see.. 
Also, wouldn't it be strange to sell boxes of not that common food additives like that?
So I added sodium carbonate to all the solutions (I will number them from 1st - the biggest bag and 3rd the smallest), 1st and 2nd did nothing and 3rd
did the bubbling.
Did some Russian research and got that sodium bisulfite is "Гидросульфит натрия" and there is that "o" still
visible.
Ok, so we have:
Sodium bisulfite
metabisulfite (smallest bag)
Ammonium chloride.
I will figure out which is which! 
This one was also easy.  too easy! too easy! 
Next one coming soon!
[Edited on 3-8-2016 by TheMrbunGee]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Ok, so if it's "osulfate" then you probably have sodium thiosulfate. Here's what I would do:
-Take a bit of each of the substances, and mix them as powders with solid sodium hydroxide.
-Add a few drops of water to each of the piles. The one that is ammonium chloride should react and produce ammonia gas, while the others do nothing.
-Dissolve a bit of each of the remaining two in water in separate test tubes. Add some dilute HCl. Both should react to produce sulfur dioxide, but
there will also be a fine yellow precipitate of elemental sulfur in the one that is thiosulfate.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
You can rule out bisulphate by reacting it with a solution of sodium carbonate; the evolution of CO2 as bubbles will confirm the acidic
properties of the bisulfate.
You can rule out the metabisulfite by reacting your powders with oxidants like
- Permanganate: starting from an acidified solution of your X salt, add small drops of a diluted solution of KMNO4. If the purple drop gets
discoloured, it means the permanganate got reduced, indicating the presence of the sulfite.
- Iodine/iodide: partially oxidize potassium iodide to iodine with few drops of hydrogen peroxide (you will get a brown solution which is
I3-) then add a few drops of the iodine/iodide mix to a solution of your could-be-sulfite and see if it gets discoloured from
brown to pale yellow, indicating the reduction of I2 to I-.
- Potassium cromate/dicromate: starting from an acidified solution (H2SO4) of cromate/dicromate, add a few drops of your
could-be-sulfite solution; the reduction of orange/red Cr (VI) to green Cr (III) will indicate the presence of sulfite.
These reactions will not interfere with the other 2 compounds.
You can rule out the ammonium chloride by reacting your powders with a solution of NaOH. The cringe smell of ammonia should be enough. Alternatively,
if you have access to phenol, you could try the colorimetric method (phenol + sodium hypocloride) which will yield a blue solution.
Otherwise, if you can confirm the first 2, then by exclusion the 3rd will be the chloride (which you can further confirm by precipitating the
Cl- with AgNO3).
If the first is not bisulphate but neutral sulphate, you can precipitate it with barium chloride (even though this might interfere with the
metabilsulfite, if you haven't ruled it out yet)
These methods should provide instant results.
EDIT: I'm at work so I was too slow to reply.
If it's sodium bisulfite, it will get oxidized the same way the metabisulfite does. Since you said that the 3rd bag (which is metabisulfite) reacted
with the carbonate, I suppose I overlooked the acidic properties of the metabisulfite (even though the oxidation tests would have ruled it out from
bisulphate anyways). See if one other of the first 2 bags gets oxidized and see if the remaining one precipitates AgCl.
[Edited on 3-8-2016 by Metallus]
[Edited on 3-8-2016 by Metallus]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | You can rule out bisulphate by reacting it with a solution of sodium carbonate; the evolution of CO2 as bubbles will confirm the acidic
properties of the bisulfate.
|
I did the tests and:
Big bag - sodium thiosulfate - sulfur precipitated with HCl
Middle bag - ammonium chloride - ammonia released with NaOH
Small bag - Sodium metabisulfite (acidic nature)
Thank You guys! 
Still - interesting combo to sell in one box.. :?
Next chemical looks like to be CaCO3, could not dissolve anything in 400ml 100C water. Also reacts with acids and releases CO2. So I think I got it
and now start to prepare next one!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by TheMrbunGee  | Next chemical looks like to be CaCO3, could not dissolve anything in 400ml 100C water. Also reacts with acids and releases CO2. So I think I got it
and now start to prepare next one!
|
You're probably right, but it could be another alkali earth carbonate. To be sure, add some sulfuric acid
or a soluble sulfate salt to the solution dissolved in HCl. If you don't get a precipitate, it's probably actually magnesium carbonate. If you do,
you're probably correct about calcium carbonate, but to verify it though, you could do a flame test with some of the HCl solution on a wood splint. If
it's orangish, it's probably calcium, but if it's bright red then it's really strontium carbonate, and green would indicate barium carbonate.
[Edited on 8-3-2016 by zts16]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by zts16  | Quote: Originally posted by TheMrbunGee  | Next chemical looks like to be CaCO3, could not dissolve anything in 400ml 100C water. Also reacts with acids and releases CO2. So I think I got it
and now start to prepare next one!
|
You're probably right, but it could be another alkali earth carbonate. To be sure, add some sulfuric acid
or a soluble sulfate salt to the solution dissolved in HCl. If you don't get a precipitate, it's probably actually magnesium carbonate. If you do,
you're probably correct about calcium carbonate, but to verify it though, you could do a flame test with some of the HCl solution on a wood splint. If
it's orangish, it's probably calcium, but if it's bright red then it's really strontium carbonate, and green would indicate barium carbonate.
[Edited on 8-3-2016 by zts16] |
I heated it a lot (to more than 1000 C) so it is not MgCO2. But you are right! I dissolved it in HCl and flame test gave a red color to the flame..
Could it also be Li2CO3 :?
And why wiki says that SrCO3 is hydroscopic? is it? If it is then mine is Li2CO3. Because my powder has been standing outdoors for years and it has
not lumped up
[Edited on 3-8-2016 by TheMrbunGee]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Well, is the sulfate soluble in water, or not? Lithium sulfate is soluble, strontium sulfate is not.
Compounds can be hygroscopic without being deliquescent, as anhydrous hygroscopic solids can absorb water from the air, without necessarily
liquifying. Think of magnesium sulfate or sodium carbonate.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by zts16  | Well, is the sulfate soluble in water, or not? Lithium sulfate is soluble, strontium sulfate is not.
Compounds can be hygroscopic without being deliquescent, as anhydrous hygroscopic solids can absorb water from the air, without necessarily
liquifying. Think of magnesium sulfate or sodium carbonate. |
Then it is SrCO3.
Yes, but MgSO4 clumps up, just like NaCO3.. This one is nice freeflowing powder, not even small cumps.. strange.. 
ok, soon I will prepare next one! 
So the next one is kind of ready!
These are colorless crystals.
There is a huge endothermic solubility in water. Insoluble in acetone. Melting point is 100C-200C (low) and liquid boils leaving
smoke (no significant odor) until nothing is left.
Solutions and crystals was reacted with:
HCl nothing
H2SO4 dissolves good and fast, gets really hot, but nothing changes. (Picture below)
NaOH nothing
CuSO4 nothing
SnCl nothing
Al nothing (mixed and heated strongly)
KNO3 nothing (mixed and heated strongly)
Sugar nothing (mixed and heated strongly)
Iodine nothing (mixed and heated strongly)
Phosphate test negative
HNO3 small amount of xsalt and ~1ml of conc. HNO3 was mixed. White paste formed (something less soluble formed) when I added more
water - everything dissolved and I was left with clear solution.
Nothing much, but something!

Xsalt itself!

H2SO4 and xsalt after cooling. (Plastic container melted from the heat)
[Edited on 4-8-2016 by TheMrbunGee]
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
I would have said NH4NO3 but some non-reactions leave me wondering.
I'll go have lunch now, I'll edit later.
- - - - -
Ok I'm back.
NH4NO3 melts at 150-200°C and decomposes into water and nitrogen. Its endothermic reaction with water is used in the
instant-ice bags (which usually contain NH4NO3 and a few ml of water contained in a thin bag that breaks when you bend it).
You won't get any appreciable reaction with acids, but when you mix it with NaOH I would expect to smell some ammonia. Can you smell anything when you
mix the two?
The low solubility in conc HNO3 is common to many other nitrates.
When you mix it with H2SO4 I would expect the formation of HNO3 and perhaps some NOx. Can you appreciate
any faint yellow tint? I remember that when I mixed H2SO4 with KNO3, the solution would release some heat, but not
enough to melt a plastic cup.
If it is indeed a nitrate, you could try to add it to an acid solution of iodide or Fe(II) or sugar and see if something happens. Preferably use
HCl/CH3COOH to acidify, as other oxidizing acids will interfere.
However that appearance... AN is white, yours are transparent crystals with a yellow tint.
You could perhaps try adding a little amount of AgNO3; if nothing precipitates, then it might indeed be a nitrate. If something
precipitates, then it could be the chloride (but you would be able to smell the HCl and ammonia released on heat, so I doubt it).
[Edited on 4-8-2016 by Metallus]
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
does the mystery compound have an odour ?
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  |
NH4NO3 melts at 150-200°C and decomposes into water and nitrogen. Its endothermic reaction with water is used in the
instant-ice bags (which usually contain NH4NO3 and a few ml of water contained in a thin bag that breaks when you bend it).
You won't get any appreciable reaction with acids, but when you mix it with NaOH I would expect to smell some ammonia. Can you smell anything when you
mix the two?
The low solubility in conc HNO3 is common to many other nitrates.
When you mix it with H2SO4 I would expect the formation of HNO3 and perhaps some NOx. Can you appreciate
any faint yellow tint? I remember that when I mixed H2SO4 with KNO3, the solution would release some heat, but not
enough to melt a plastic cup.
If it is indeed a nitrate, you could try to add it to an acid solution of iodide or Fe(II) or sugar and see if something happens. Preferably use
HCl/CH3COOH to acidify, as other oxidizing acids will interfere.
However that appearance... AN is white, yours are transparent crystals with a yellow tint. 100% sure all this stuff is inorganic?
[Edited on 4-8-2016 by Metallus] |
It may be organic..
With NaOH cant smell ammonia and with H2SO4 it doesnt smell like HNO3. I don't think it is nitrate, because it is not oxidant. no reaction with sugar
nor with Al.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TheMrbunGee  | Quote: Originally posted by Metallus  |
NH4NO3 melts at 150-200°C and decomposes into water and nitrogen. Its endothermic reaction with water is used in the
instant-ice bags (which usually contain NH4NO3 and a few ml of water contained in a thin bag that breaks when you bend it).
You won't get any appreciable reaction with acids, but when you mix it with NaOH I would expect to smell some ammonia. Can you smell anything when you
mix the two?
The low solubility in conc HNO3 is common to many other nitrates.
When you mix it with H2SO4 I would expect the formation of HNO3 and perhaps some NOx. Can you appreciate
any faint yellow tint? I remember that when I mixed H2SO4 with KNO3, the solution would release some heat, but not
enough to melt a plastic cup.
If it is indeed a nitrate, you could try to add it to an acid solution of iodide or Fe(II) or sugar and see if something happens. Preferably use
HCl/CH3COOH to acidify, as other oxidizing acids will interfere.
However that appearance... AN is white, yours are transparent crystals with a yellow tint. 100% sure all this stuff is inorganic?
[Edited on 4-8-2016 by Metallus] |
It may be organic..
With NaOH cant smell ammonia and with H2SO4 it doesnt smell like HNO3. I don't think it is nitrate, because it is not oxidant. no reaction with sugar
nor with Al. |
If it is organic, dump it inside 2M H2SO4 + dichromate mix. That shit will oxidize almost anything. If you have permanganate,
all the better.
A more economic version can also be H2O2 in NaOH.
If it readily oxidizes or burns, we can rule out some common inorganic alternatives.
[Edited on 4-8-2016 by Metallus]
[Edited on 4-8-2016 by Metallus]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  |
If it is organic, dump it inside 2M H2SO4 + dichromate mix. That shit will oxidize almost anything. If you have permanganate,
all the better.
A more economic version can also be H2O2 in NaOH.
If it readily oxidizes or burns, we can rule out some common inorganic alternatives.
|
when melted reacts with magnesium powder and emits ammonia gas!
I mixes xsalt with water and KMnO4 and slowly added conc. H2SO4 after a bunch of drops some gas started to evolve. I put a burning match to the
bubbles and it went off. (N2, CO2)
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
It really sounds like ammonium nitrate to me. In acid KMnO4 it decomposes to release water and ammonia. Does the reaction yield a
brown/black precipitate (MnOx) ?
Please, try to perform one of the reductions I listed above.
| Quote: | | If it is indeed a nitrate, you could try to add it to an acid solution of iodide or Fe(II) or sugar and see if something happens. Preferably use
HCl/CH3COOH to acidify, as other oxidizing acids will interfere. |
I remind you that an acid medium is required to help the nitrate oxidize. Just dumping Al or sugar to a neutral solution of nitrate won't do a thing.
Try dissolving your salt in HCl and then perform again those reactions. Heat if necessary.
Alternatively, add your salt to a 1:1 diluted H2SO4 and then drop some copper in it. HNO3 readily oxidizes copper to
copper nitrate. If you see red fumes and a blue solution, then that's it.
Again, another quick test is adding a little amount of AgNO3 (expensive). Most anions will precipitate but NO3-.
Sorry if I'm fixated with AN, but I just can't think of another inorganic salt with those properties (the low melting point and evolution of odorless
gases coupled with the highly endothermic reaction with water really scream AN to me).
[Edited on 4-8-2016 by Metallus]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | It really sounds like ammonium nitrate to me. In acid KMnO4 it decomposes to release water and ammonia. Does the reaction yield a
brown/black precipitate (MnOx) ?
Please, try to perform one of the reductions I listed above.
| Quote: | | If it is indeed a nitrate, you could try to add it to an acid solution of iodide or Fe(II) or sugar and see if something happens. Preferably use
HCl/CH3COOH to acidify, as other oxidizing acids will interfere. |
I remind you that an acid medium is required to help the nitrate oxidize. Just dumping Al or sugar to a neutral solution of nitrate won't do a thing.
Try dissolving your salt in HCl and then perform again those reactions. Heat if necessary.
Alternatively, add your salt to a 1:1 diluted H2SO4 and then drop some copper in it. HNO3 readily oxidizes copper to
copper nitrate. If you see red fumes and a blue solution, then that's it.
Again, another quick test is adding a little amount of AgNO3 (expensive). Most anions will precipitate but NO3-.
Sorry if I'm fixated with AN, but I just can't think of another inorganic salt with those properties (the low melting point and evolution of odorless
gases coupled with the highly endothermic reaction with water really scream AN to me).
[Edited on 4-8-2016 by Metallus] |
It is not ammonium nitrate. 100% not. I have worked with AN, and this does not do things AN does.
To be extra sure I added H2SO4 to the salt and added copper. Nothing happens, mixture doesn’t smell like nitric acid or anything and does not react
with Cu. not at all! Not when concentrated and not diluted.
I did some more tests and it is urea. 
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Wow, I was oblivious of urea's endothermic reaction with water.
I have only studied it from theory, never actually got to use it in lab, didn't know it had these properties as well. I am now reading that certain
cold packs also contain urea instead of AN.
One more thing learnt.
Keep them coming, I'm having fun.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | Wow, I was oblivious of urea's endothermic reaction with water.
I have only studied it from theory, never actually got to use it in lab, didn't know it had these properties as well. I am now reading that certain
cold packs also contain urea instead of AN.
One more thing learnt.
Keep them coming, I'm having fun. |
Good to know! 
OK, a trick questeon - What the f... can I do with about 8 kg of MgSO4 * 7H2O??!?
I had these 6 jars as unidentified compounds, they all looked similiar and I did quick tests with NaOH and they all are magnesium sulfate. that
spoiled my excitment. :/
I think I will dispose them and leave me with about 2 kg max.

|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
That's Epsom salt!
If you are "clogged" down there, eat a tablespoon of it and you'll shit demons.
Btw, have you performed other tests to be sure it's MgSO4 and not some other compound of a polyvalent cation? Redox reactions are usually a nice way
to rule out common salts (carbonate, sulphate) of alkaline metals/earths.
[Edited on 5-8-2016 by Metallus]
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
You can grow huge polycrystal 
Nah, you can use it as fertilizer I think and I heard someone is using it to prevent animals from nibbling trees. I don't think it has many uses since
it's already hydrated.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
the jars are more valuable than their contents, but a little as fertiliser, some as bath-salts?
better to use than dispose.
re-crystalise some for stock, air-drying gravity filtered is tedious, definitely use vacuum if available.
Anhydrous MgSO4 seems to store quite well in a jam jar, maybe roast a little?
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | That's Epsom salt!
If you are "clogged" down there, eat a tablespoon of it and you'll shit demons.
Btw, have you performed other tests to be sure it's MgSO4 and not some other compound of a polyvalent cation? Redox reactions are usually a nice way
to rule out common salts (carbonate, sulphate) of alkaline metals/earths.
[Edited on 5-8-2016 by Metallus] |
Yes, it is MgSO4! 
So next one (I think its last one) is Chromium compound. I sits in a jar, that says it is Cr2(SO4)3, but wiki says, that Cr2(SO4)3*xH2O is purple.
Mine stuff is green, it is soluble in water and near 100C become honey like gue. Solutions react with solutions of NaOH, precipitating greenish stuff
(color might be because of solution color.).

Weak solution of salt.
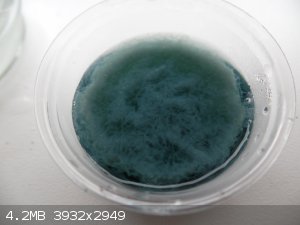
Precipitate from reaction with NaOH solution.

This formed when concentrated CrXsolution was added to concentrated solution of NaOH
Edit:
Cr2(SO4)3*15(H2O) is green!
Solutions react with Mg powder.
[Edited on 5-8-2016 by TheMrbunGee]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by TheMrbunGee  | Quote: Originally posted by Metallus  | That's Epsom salt!
If you are "clogged" down there, eat a tablespoon of it and you'll shit demons.
Btw, have you performed other tests to be sure it's MgSO4 and not some other compound of a polyvalent cation? Redox reactions are usually a nice way
to rule out common salts (carbonate, sulphate) of alkaline metals/earths.
[Edited on 5-8-2016 by Metallus] |
Yes, it is MgSO4! 
So next one (I think its last one) is Chromium compound. I sits in a jar, that says it is Cr2(SO4)3, but wiki says, that Cr2(SO4)3*xH2O is purple.
Mine stuff is green, it is soluble in water and near 100C become honey like gue. Solutions react with solutions of NaOH, precipitating greenish stuff
(color might be because of solution color.).
Weak solution of salt.
Precipitate from reaction with NaOH solution.
This formed when concentrated CrXsolution was added to concentrated solution of NaOH
Edit:
Cr2(SO4)3*15(H2O) is green!
Solutions react with Mg powder.
[Edited on 5-8-2016 by TheMrbunGee] |
Yeah, the colour depends on the degree of hydration.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Strange that it does not hydrate to the maximum in water solution.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
found one more that confuses me!
The jar says it is Zinc borate (metaborate) with formula Zn(BO2)2, but I cant find anything like that on google.
It is fine white powder. does not dissolve in water, but does in diluted H2SO4.
|
|
|
| Pages:
1
2
3
4
5 |