nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
Ester enolate
so i was looking about some reaction
i saw this
Ester Enolates
i was wondering what is the typical yield for this kind of reaction
and if the X could a chlorine molecule
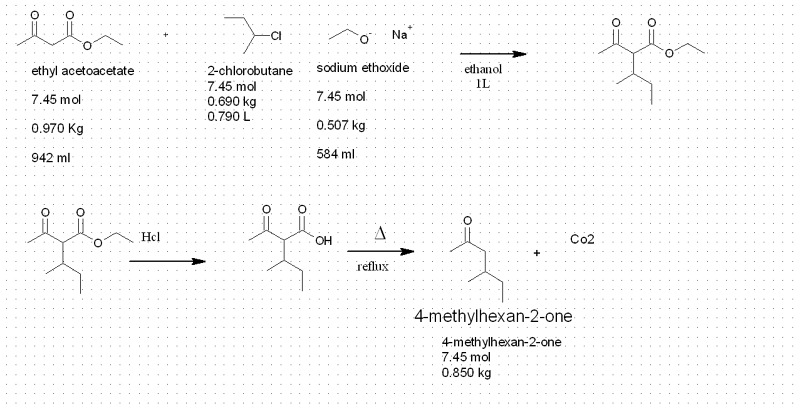
such as this reaction
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
maybe
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
As written, this will likely be a poor yielding reaction overall as you are proposing to alkylate a secondary (and not all that reactive) anion with a
secondary alkyl chloride. This first step will really poorly impact your overall yield. Switching to an iodide from the chloride will probably help a
fair amount but the alkylation yield will still not be anywhere near quantitative. It would also help to use a fair excess (>50%) of the iodide to
help things along. Finally, the alkylation reaction as posted is VERY concentrated. This will probably result in a hard-to-manage slurry at some point
during the process. All that being said, you will no doubt be able to obtain your desired ketone if you can isolate the intermediate alkylated keto
ester. Those last steps are a relative piece of cake.
AvB
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
Sec-butyl ethyl ether will be produced and your base will attack the ethyl acetoacetate.
To improve the scheme use 2-iodobutane as suggested by AvB and reduce your quantities down adding base gradually.
Atropine, Bicarb, Calcium.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I've never done any acetoacetic ester syntheses, but I have done a malonic ester synthesis (using a primary alkyl bromide, and anhydrous potassium
carbonate as a base). One cause of lower yield was formation of dialkylated product. However, this might be less likely to happen with a relatively
unreactive secondary alkyl chloride.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
using Cl ,there can be both O and C alkylation -http://pubs.acs.org/doi/abs/10.1021/jo01258a101
better to use Br or I
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by Metacelsus  | | I've never done any acetoacetic ester syntheses, but I have done a malonic ester synthesis (using a primary alkyl bromide, and anhydrous potassium
carbonate as a base). One cause of lower yield was formation of dialkylated product. However, this might be less likely to happen with a relatively
unreactive secondary alkyl chloride. |
If you can deprotonate your acetoacetate with carbonate, do so. Carbonate won't compete for the alkyl bromide. It may be slower, but that's better
than getting nothing.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Also, try using the 2-chlorobutene or 2-bromobutene instead, it will be a much hotter alkylating agent. You could then reduce it back to the
saturated group later. I have done almost that exact alkylation before that way. The alkyl was very hard to get any reaction, but the haloalkene
was much more reactive. Adds a step, but works well. Just a suggestion.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Dr.Bob,
I assume you are referring to 2-halo-3-butene (the allylic derivative) and not the vinylic halide.
AvB
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Yes, I was low on coffee at the time. I think I used the 2-chloro-3-methyl-3-butene, which is symetrical, thus giving only one major product,
whereas the 2-halo-3-butene can give both isomers, due to allylic reactivity. It's been a long time since I tried that, so I barely remember the
details now, unfortunately. But halo allyl groups are much hotter akylating agents.
|
|
|