aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ridding Apparatus of Water
Having seen many references to 'dryness' in write-ups (e.g. Grignards) it seems that there is no standard way to get rid of the majority of
atmospheric moisture from glass apparatus.
Some say to heat it all with a blowtorch, others say oven-dry, yet others say to purge with dry gas.
I imagine that purging with warm, dry air would suit most amateurs, as the notion of whacking a blowtorch flame onto precious glassware would be, er,
unplatable, and the home oven may not be available for such uses.
This brings us to How the air can be dried.
In the Amateur setting there exist many substances that can be used as drying agents, such as Sand, Dry Rice, Table Salt, NaOH prills,
CaCl2, maybe even molecular sieves or highly concentrated suphuric acid.
I suggest a series of simple experiments to establish the efficacy of each available substance that anybody can participate in, the results of which
would help to empirically determine the performance of each substance.
As a suggestion, the substance to be tested is prepared according to some documented procedure (document it yourself if none are on hand) such as
oven-drying table salt for 1 hour at 200 C, or heating/stirring sand in a beaker for 20 minutes.
Some of this is loaded into a 24/27 extension tube, with cotton-wool used to 'plug' it in place, the whole unit being weighed (s).
1-hour oven dried @ 200C CaCl2 can be used as a reference, some being placed in a second tube, similarly packed with a small amount of
cotton wool, then likewise weighed (r).
The two tubes are placed in-line with each other, then a small pump of any kind used to push air through the system for 't' seconds.
(alternatively, each sample could be stuffed into a 'sock' of filter paper, then shoved into each end of a liebig condenser)
The flow-rate 'f' of the pump used can be pre-determined simply by blowing air into an inverted measuring cylinder in a bowl of water for a set time.
By measuring the weight change of each unit, empirical data can be gathered from which a proper comparison can be made of each drying agent.
If the ambient humidity can be measured then the accuracy will be enhanced, although if a series of tests are done in a short time, the relative
differences should also provide useful data.
The specifics of how this experiment could/should be done are very much wide open to suggestions, ridicule, hopefully discussion.
The aim is to find out how available substances will work best for amateurs trying to exclude atmospheric moisture from their apparatus.
[Edited on 26-9-2016 by aga]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
For Grignards you don't really have to worry about drying the air. You just have to boil your ether or THF for a while prior to adding the alkyl
halide, that way the apparatus is filled with solvent vapors. Stick a drying tube at the top of the condenser and then you'll be sure that any air
that gets in once the ether cools down is dry. I ran one last weekend when the humidity was 76% and it worked just fine. If you're going for
super-ultra-maximum yields then sure you can oven or flame dry the glassware but I feel like that's a bit excessive unless the reagents you're using
are incredibly expensive and you need to have a great yield or else waste a lot of money.
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
I was taught that the surface of glass adsorbs a considerable amount of moisture, and that heating at 200C was needed for scrupulously dry glassware.
That and either assembling hot or letting it cool in a desiccator.
I've done some moisture sensitive reactions without this with success, but never anything really finicky Like those Gilman reagents. Maybe with
proper procedures you wouldn't need an iodine crystal to get your grignard going, but it sound a lot easier to me to use the iodine.
I do bake my glassware at 200C for dry condition reactions, but I just let it cool in the oven till I can handle it. I don't know if this would help
at all if you used a gas oven.
Well, to be honest I'm too cheap to buy a big enough desiccator to fit my condenser in there for it to cool off anyway. Maybe some day when I've taken
care of about 25 other priority items for my lab.
EDIT In re post right after mine: Ziqquratu, thanks Hadn't thought of that. I'll go look for some Tupperware that'll fit my condensers etc, I've
already got plenty of cake racks.
[Edited on 27-9-2016 by Maroboduus]
[Edited on 27-9-2016 by Maroboduus]
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
How dry you need your equipment depends on what you're trying to do. Some reactions need to be fairly dry, but are still tolerant of a little water -
with Grignards being the classic example where a bit of water won't bother anyone. On the other hand, some reactions genuinely require scrupulous
technique in a glove box with water and oxygen scrubbers and recirculated atmosphere and...
For most purposes, I dry my glassware in the oven at 110-130*C for a minimum of 1 h (it's fairly arbitrary, and since I have the luxury of an
always-on lab oven, I usually try to keep a selection of glassware in there for use as I need it). I then seal it and allow it to cool under nitrogen;
you can also place it in a desiccator (I use silica gel in mine) and assemble it after it has cooled, or use a drying tube to dry the air as it enters
(with CaCl2 usually being the desiccant of choice).
Maroboduus, you don't necessarily need a "real" desiccator - you could just buy a sealable plastic container and a heatproof base of some sort (a cake
rack would work well).
For extra-dry reactions, flame drying is easy enough - set up your apparatus, allow dry gas to flow through it, and heat with a blowtorch or a
heatgun, making sure to keep the torch/gun moving continuously - both to prevent melting the glass, and to ensure even and complete heating. The heat
gun is safer, because they generally can't get hot enough to melt borosilicate glass; the torch is fine if you don't stop in one spot too long. I
generally flame dry for about 10 minutes to be sure all water has been expelled.
For drying a flow of gas, aga, a lot of the work has been done - we use silica gel, CaCl2, H2SO4, and NaOH fairly
routinely, and they all work fairly well. Of course, if you can start with fairly dry gas for your experiments, you're a long way ahead - if you're
planning a lot of dry work, a cylinder of welding nitrogen is pretty cheap and will go a long way! But, that said, don't let me discourage you from
experimenting! It sounds like you've got a reasonable protocol in mind; perhaps if you could find a way to measure both inlet and outlet humidity, as
well as ensuring a consistent flow rate, it might improve the utility of your results.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
There is always the glove box, but...
When I'm really worried about water, especially that adsorbed to glass, I'll pull a vacuum on the apparatus (a Schlenk line is great for this--it also
allows for testing how air-tight your apparatus actually is), flame it with a bunsen burner (you can watch the desorbed water move up, sometimes as a
ring), purge with inert gas (N2 or Ar) while cooling and thereafter, and either maintain purge gas or put a drying tube/train on there.
Honestly, this rigor can frequently be overcome by the use of a slight excess of reagent. Hell, it has even been found that some sensitive reactions
don't go without a tiny catalytic amount of water (any more kills them), so rigorous drying might not always be for the best.
It really depends on the reaction you want to do and what purification strategy you are planning to use.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Testing drying setups is tough, as everyone starts at a different place, due to variations in temperature, humidity, scale, and solvents. What works
in Boston during the winter might not work in Florida in the summer. For most cases, either oven drying or flame drying will work, and as stated,
many reactions are just not that sensitive, but some are. For larger scale, often it is not as bad, as the surface area to volume of the glass is
lower, and dry inert gases are often available. Just blowing dry N2 through a rbf will remove a lot of moisture, especially if you heat it some. I
often use "dry" solvent to rinse the flask to remove most of the remaining water.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The intention is/was to test substances on-hand to evaluate their effectiveness in drying air, which is something i'll do in the near future.
CaCl2 will probably come out on top, although i have no idea by what margin over, say, dry rice.
[Edited on 29-9-2016 by aga]
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If you place glasswware like round bottomed flasks in an oven to dry you must, when removing from oven, suck the contents of the flask out with a
vacuum cleaner and a narrow tube as the air inside can be very moist and will condense on the flask when it cools.
Nafion tubing can be used to dry any gas I have read.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
This may help...
Common lab dessicant information page out of Merck Chemical Lab Memento book...
Set by order of efficiency...
CaCl2 is into the third position
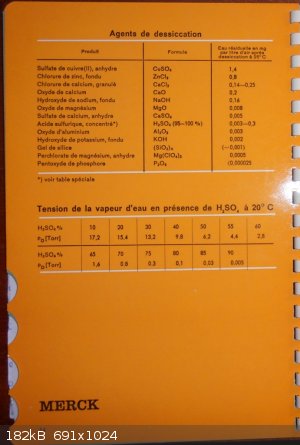
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
In school we were taught when prepping for Grignard, rinsing with acetone after lab detergent hot water and air dry with compressed air.
Would only work if air was dry as well? or was this method mostly for cleaning? Been a while lol.
There is nothing more useless than doing well that which need not be done at all.
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Quote: Originally posted by PHILOU Zrealone  | This may help...
Common lab dessicant information page out of Merck Chemical Lab Memento book...
Set by order of efficiency...
CaCl2 is into the third position
|
I don't actually speak french, but that looks backward to me.
Phosphorus pentoxide at the bottom?
Also, although I admit I don't speak French, the tittle for the column looks to me like it says something about remaining water in Mg per liter.
(after desiccation)
Am I mistaken here?
[Edited on 27-11-2016 by Maroboduus]
EDIT, but I had no idea silica gel was so complete as a desiccant.
[Edited on 27-11-2016 by Maroboduus]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Maroboduus  | Quote: Originally posted by PHILOU Zrealone  | This may help...
Common lab dessicant information page out of Merck Chemical Lab Memento book...
Set by order of efficiency...
CaCl2 is into the third position
|
I don't actually speak french, but that looks backward to me.
Phosphorus pentoxide at the bottom?
Also, although I admit I don't speak French, the tittle for the column looks to me like it says something about remaining water in Mg per liter.
(after desiccation)
Am I mistaken here?
[Edited on 27-11-2016 by Maroboduus]
EDIT, but I had no idea silica gel was so complete as a desiccant.
[Edited on 27-11-2016 by Maroboduus] |
Lucky you, I speak French 
Yes P2O5 is a strong dehydratant.
Into the list also silica gel ("Gel de silice")...
I hope you enjoy Gypsum (plaster of Paris as CaSO4 anhydrous = Drierite).
Sadly they don't list Ba(ClO4)2 an old lab dessicant (I own two bottles of it...but clearly I will not use it to dry stuffs but wel to make
perchlorates and energetic materials.
They mention the residual moisture into the air above the solid (most likely into a closed vessel like a dessicator) --> mg/L (not Mg/L Mg=
Magnesium and transmutation is not into question here  ) )
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Dwarvensilver  | In school we were taught when prepping for Grignard, rinsing with acetone after lab detergent hot water and air dry with compressed air.
Would only work if air was dry as well? or was this method mostly for cleaning? Been a while lol. |
Compressed air is a terrible thing to dry glassware with, since it's usually contaminated with water (which condenses when the air is pressurized) or
oil (from the pumps). When I was at university, all the undergrad labs had both compressed air and vacuum on tap. The vacuum was perfect for drying
glassware, but a significant portion of my students insisted on using the compressed air, making shrieking noises and ruining their experiments. I
should have failed them on principle.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
In my current (non-hobbyist) lab, I routinely do air and moisture sensitive reactions with finicky organometallic catalysts. Flame drying with a
Bunsen Burner has always worked well for me. After heating, let it cool down in a 110 - 130 C oven, then assemble the apparatus hot. I've not yet
melted borosilicate glassware this way.
Oven drying works perfectly fine too. But flaming the glass and watching the condensation migrate out of a flask just feels reassuring.
I'm skeptical of simply purging with hot, dry gas. Then again, it's just not something I ever do. Flaming takes 10 seconds and I've done it hundreds
of times.
| Quote: | | In school we were taught when prepping for Grignard, rinsing with acetone after lab detergent hot water and air dry with compressed air.
|
Just be careful about removing acetone residues lest they react with your Grignard.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Thanks for the tips gentlemen, the learning that goes on here and the knowledge base that is available is bloody amazing.
DDTea do you mean short of totally dry or that acetone molecules have an affinity for glass?
Dwarven
There is nothing more useless than doing well that which need not be done at all.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
I mean dry your glassware completely after chasing the water away with acetone. It won't stick to your glass.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|