| Pages:
1
2 |
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
The art of crystallization
so not long time ago i got this idea of using a coffee thermocan for maximizing cooldown stability, which i had an idea of would give me better luck
with forming big crystals
so i followed the idea, and as im currently messing about with ammonium phosphate i tossed a hot solution of impure ammonium phosphate into a thermo
coffee can along with a single seed crystal approx 2mm big
opened the thing and i found 2 big crystals in there, one of them as big as FOURTY MILLIMETRES weighting just about 15g, supposedly mono-di ammonium
phosphate
http://puu.sh/rqGrd.jpg
im thinking about constructing an even better container, aerogel seems to be the best option of insulation, styrofoam scoring just around 4 in
insulation, there is also polyurethane which can be around 6, but aerogel up to 12, but for insulation its impotant to have a proper lid to make it
airproof which suddenly makes this whole idea more difficult to go ahead and make
in short.. i found the holy grail for crystallization, how would one go about reaching higher insulation efficiency along with more capacity?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Aerogel would be good for insulation since it's mostly air. Even better of course would be to remove the air entirely, and use a vacuum insulated
flask.
What do you mean when you say Styrofoam is a "4", etc.? What is that number based on?
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
https://en.wikipedia.org/wiki/R-value_(insulation)
yes vacuum has up to 50 in r-value.. but im thinking its gonna first up be expensive to get anywhere near, im looking for widemouth screwlid
container, and that would probably come without insulation if its even possible to find, so i think it would lose a lot of efficiency on the lid
itself
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
You could also put a large thermal mass around the crystalisation vessel then insulate that.
Water forms a good thermal mass.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
the concept would work, but practically it would be a mess having to heat up x litres of water and weight the container down, very slow cooling also
works wonders to get very pure crystals as less impurities gets trapped in the crystals so not only as a smaller experiment once a week this would be
desired to be used but many times in a day, now using this technique for purification would be more doable if a big container able to fit several
beakers or likewise inside of it was possible
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
They make vacuum flasks for soup with a wide mouth, the most popular brand here in Aus are made by Thermos http://www.thermos.com/product_catalog.aspx?CatCode=FOOD
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
thermos seems to be limited to 1L
maybe a widemouth thermocan could be used then the screwlid part could be stucked together with a larger homemade insulated chamber?
what would be important for a screwlid would be it being able to be very airproof once sealed as that plays a major role in keeping the heat inside of
the container
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Hydroflask makes 64oz (almost 2 liter) insulated bottles. These things can keep ice water icy for several consecutive days. They're really good. Only
issue is that it may be difficult to get larger crystals out of the mouth of the bottle.
https://www.rei.com/rei-garage/product/108988/hydro-flask-wi...
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
An oven with a thermostat can be cooled down at a controlled and well-defined rate, and the total dimensions could probably be made smaller than a
solution requiring a lot of insulation and thermal mass.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Quote: Originally posted by phlogiston  | | An oven with a thermostat can be cooled down at a controlled and well-defined rate, and the total dimensions could probably be made smaller than a
solution requiring a lot of insulation and thermal mass. |
I also think that's a best way to go.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2659
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
You could use foam in a can and insulate nearly any container, or even build a box of 4" styrofoam, then put any container in the middle and foam the
remaining space to save money. I have done resolution crystallizations using an oil bath before, and they are a nightmare to keep consistent, the
unevenness of an oven or oil bath can lead to terrible conditions, much better to use a well insulated container, where there is little convection or
conduction in the liquid. If I had it to do over, I would build a giant thermos for it. Or use a large Dewar flask.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
im thinking about just getting a gigantic brick of styrofoam and then ramming some wooden box around it for durability, but again im thinking the
pressure from the hot solution could be a problem, most crystallizations dont require much past 100*C so a metal plate on the bottom of this hollowed
out styrofoam brick would probably not even be necessary, wood would be a good alternative tho, low heat capacity
styrofoam would be very difficult with solvents such as acetone however, maybe the idea for this styrofoam box would simply be to get a HDPE container
with screwlid, widemouth and then stuff it into this styrofoam brick, then make a lid with styrofoam glued to a wooden plate and make it so that you
can tighten the lid onto the sides of the box?
now an oven would indeed be the best, but that would crave a bit of insulation as well, mineral wool would work for that, another problem with an oven
would be making sure it doesnt actually fluctuate in between temperature but rather very smoothly transist in temperature, i suggest maybe a water
bottle with heater and thermostat, then insulated box for this, the water would then deliver the temperature difference SMOOTHLY with rather low heat
radiation -- low heat fluctuation
mineral wool would be ideal if were talking very hot heating elements such as in regular ovens or toasters
for an easy solution hydroflask seems to be a very great choice
maybe for a styrofoam box one could create vacuum that would keep the box together, supposing that wouldnt mess up the whole crystallization process
somehow, maybe a closed container would be required for that as the water vapour would fill out the vacuum?
what really got my attention about ammonium phosphate was that seemingly the tri-ammonium version, which is thermally unstable seems to form some
really nice prisms, i think it would be pretty rad for make one gigantic prism, as in out of naturally grown crystal -- not like quartz prism that has
been cut
http://puu.sh/rs3yG.jpg
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
If you have not already, you might enjoy seeying the impressive potassium dihydrogen phosphate crystals they grew for the optics in the National
Ignition Facility:

Descriptions of the methods that were used to grow these can be found online.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
What The Actual Flying FUCK? That is insane. And gorgeous.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
https://www.youtube.com/watch?v=l_USYub3djY
interesting, i got hold of some gigantic motor about half a year ago, crystal growth it could be used for i guess? with this scale the temperature is
a lot easier to handle, its difficult to make the whole thing drop in temperature rapidly
i think its very interesting that phosphates containing phosphor -- which is necessary to all life, has a habit of forming obelisk-like crystals
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Greets all,
I came across some 24V motors that run at 6 RPM and apparently old clock motors run at 1 RPM (I.E. second hand  ) but have not tried an old clock motor yet. ) but have not tried an old clock motor yet.
Anyways it is another way to rotate your crystals while growing and I found that it seems to make better looking single crystals.
It is somewhat simpler than trying to build a chamber like for the potassium dihydrogen phosphate crystals.
Here is a link to a video of my largest alum crystal recovering from a bad melting period spell
http://vid901.photobucket.com/albums/ac220/Dwrvnsilver/Chemi...
[Edited on 9-10-2016 by Dwarvensilver]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i did wonder about this whole spinning thingy, with the KHP crystal the whole container appeared to be spinning, solution, and even the crystal which
appeared to have been placed flat on the bottom, in which i dont really get the whole concept of spinning to give anything in addition, others than
trouble?
managed to scavenge some gigantic machinery motor weighting towards 20kg which i havent yet tried powering up yet, with some tweaking it could
probably be pretty ace for crystallization.. i see your crystal is hanging in solution while the solution and container is spinning, what do you have
to say about the spinning KHP crystal chamber tho? i really hate the idea of having a string stuck inside of the crystal itself, also the hanging
string kinda makes it more difficult with an insulated crystallization chamber
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Hi Antiswat,
I am not sure why they spin the KHP container, I assume it has something to do with getting a better formed, clearer giant crystal. I noticed in the
time lapse of the KHP crystal that it gets the spin reversed, again not sure why.
In my set up I have the crystal hanging on a 7x fishing filament which has several pounds strength and only .004", it is like hair and hard to see
inside the crystal.
The crystal is hung from a loop on the motor gear and rotates @ 6 RPM.
The pictures show it better
[img] [/img] [/img]
[img] [/img] [/img]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I tried using fishing line a few times- I found it impossible to tie without it simply untying itself. I probably needed much thinner line.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Well Draconic Acid,
I just about gave up on fishing line because of the same issue lol.
I found that if you use the first part or all of a doctors knot.
[img] [/img] [/img]
When you make a loop, pass the end through twice instead of once (bottom of knot in pic) and it usually holds pretty good.
The more times you do it the easier it becomes 
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i suggest inserting a water bubbler into the solution would also agitate it, maybe if air was blowing onto the surface it could create a circular
movement of the solution, the agitation with airbubbler immersed in solution COULD very possibly create some uneven crystallization
if you need to thin down a line a bit you could maybe run some sandpaper over it, i really hate the idea of knowing there is just a tiny bit of
something inside the crystal that is not crystal itself
maybe another idea would be an insulated box with a weak fan running to pull air in and out, i still really like the idea of forming a 40mm crystal
within 24 hours, it is a bit less passive than hanging a crystal in a beaker but my worries is that temp fluctuates too much in my room to feel
comfortable with my precious crystals being left all by themselves in such hostile environments
i do imagine if you can spin the crystallization solution in an UNEVEN pattern, if you imagine the solution will then at an uneven frequency so that
the crystal and the solution will collide, and thus making the crystallization take place faster, it may be that crystal and solution will both hit
constant still however, hm.
if i had been running their little KHP crystal project i would def had been spinning the solution itself and not entire container
for tightening a line onto crystal i think the method that would involve least line going lost in the crystal would be simply gluing it to the
crystal, epoxy or superglue or similar which would have high strenght and wouldnt require a whole lot, also taking into consideration that it would be
able to deal with being in water
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
a few thoughts;
if you spin the container, the more dense (concentrated) solution will be at the outside,
with the solution nearest to the crystal being the least concentrated,
so you will get a beautiful crystal lined container 
few temperature controllers can ramp down TEMPERATURE with no short-term rises,
tuning a pid would be boring.
I think that slowly ramping down the heating POWER would give a smoother cooling curve,
but with greater ambient temperature influence.
based only on what I have read, roughening the surface of a thread (nylon monofilament) with sandpaper to thin it down
would cause crystals to grow all along the thread.
an 'ideal' thread would either have the same colour and refractive index as the crystal
or be easily dissolved out and replaced with a filler of the same colour and refractive index.
Edit: Or maybe Fleaker could supply 1.5 um diameter Wollaston wire https://en.wikipedia.org/wiki/Wollaston_wire
[Edited on 19-10-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
A few more thoughts,
yes theoretically the density of the solution would be greater at the outside of a spinning container, but in order to make an appreciable difference
I think you would have to spin the container extremely fast to throw it out of wack to the point of only lining the inside of the container, and they
do spin the giant laser KHP crystals and it seems to work 
All temperature controllers even once on temperature will give a sine wave in the temperature as waters heat capacity is so great and controllers are
only as good as the money you throw at them lol.
yup sanding down filament will cause crystal parasites, even smooth mono-filament that I use get parasites.
I understand, being a real pick ass, not wanting to have foreign bodies in your crystals but at least when you use 0.1mm mono-filament it is nearly
impossible to see.
Spinning the crystal is quite easily done and seems to give quite good results, but I have only tried it with two crystals to date but they seemed to
grow smoother and faster that way.
I am also growing on a hotplate/stirrer set at 30°C which gives a reasonably constant 25°C temp in the growing environment, this may also speed up
the growth rate due to more evaporation. I did this due to fluctuating room temperatures.
Last night I put a tiny stir bar in a 2L beaker of alum solution and set it at 60 RPM (lowest setting) with the crystal rotating at 6 rpm as well but
this morning I has an alum crystal covered with powdered alum as the extra formed crystal particles could not settle and built up on the main crystal
from staying suspended.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Its something similar this is how they made those huge KHP cystals. The rotation is to power a turbine that circulates the solution.
From https://lasers.llnl.gov/multimedia/publications/pdfs/etr/198...
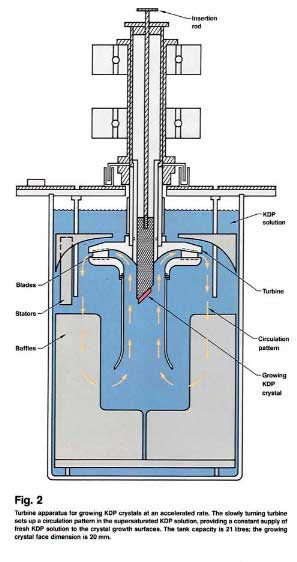
Here is a link to an other paper
https://www.cambridge.org/core/services/aop-cambridge-core/c...
[Edited on 19-10-2016 by wg48]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
This is the best paper:
https://www.researchgate.net/publication/238119403_Rapid_gro...
From the paper a continuous filter sytem
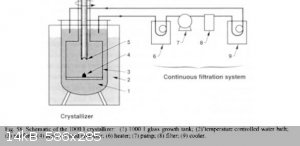
|
|
|
| Pages:
1
2 |