PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
chrysamminic acid / 1,8-dihydroxy-2,4,5,7-tetranitro-9,10-anthracenedione
I was reading Merck index in search for interesting molecular structures when I found the title compound:
chrysamminic acid / 1,8-dihydroxy-2,4,5,7-tetranitro-9,10-anthracenedione
I immediately recognised the parenthood with trinitrophenol. 
The compound is known from the early 1900.
--> Has someone informations about the detonic parameters of that compound?
It must be denser than trinitrophenol (I estimate the density to be higher than 1,8 (between 1,83 and 1,95) and display a comparable oxygen balance;
as such its detonic parameters muste be higher than TNP.
Here are some informations:
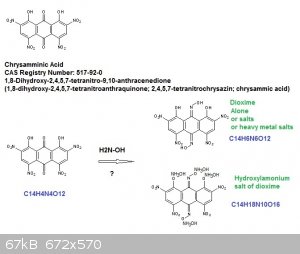
Title: Chrysamminic Acid
CAS Registry Number: 517-92-0
Name: 1,8-Dihydroxy-2,4,5,7-tetranitro-9,10-anthracenedione
Additional Names:
1,8-dihydroxy-2,4,5,7-tetranitroanthraquinone;
2,4,5,7-tetranitrochrysazin;
chrysammic acid
Molecular Formula: C14H4N4O12
Molecular Weight: 420.20
Percent Composition: C 40.02%, H 0.96%, N 13.33%, O 45.69%
Literature References:
-Prepn by heating chrysazin with fuming nitric acid
(Liebermann, Ann. 183, 193 (1876))
-Structure
(Robinson, Simonsen, J. Chem. Soc. 95, 1088 (1909))
Properties:
Golden-yellow, lustrous, bitter leaflets.
Almost insol in water; sol in alcohol with deep-red and in ether with yellow color.
CAUTION:
The acid and its salts explode when ignited or heated rapidly in air.
There is a separate tread by Bert about juglone (a natural compound found into black walnuts) in what I expose the idea to nitrate juglone...this proves that the p-quinon is quite resistant to the concentrated HNO3.
By comparing juglone and chrysazin one soon realizes the parenthood.
Chrysazin (1,8-dihydroxyanthraquinone) is used as a drug in some cancer treatment under the name Dantron.
Knowing that p-quinone dioxime is energetic despites its low OB...here follows an idea I have (comparable with the idea I had to make a dioxime of
juglone) --> chrysamminic acid dioxime.
The dioxime of chrysamminic acid must be even denser than chrysamminic acid itself (maybe reaching the 2,00 g/cm³ milestone) and displaying a similar
OB it must be even better on detonic parameters.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Philou, I came across this compound while trying to find out about another compound with a very similar "trivial" name "chrysanisic acid" that is
3,5-dinitro-4-aminobenzoic acid. Your compound can be prepared from brown Aloes resin by the action of nitric acid and this reaction is actually used
as a test for the active ingredient of the resin, barbaloin. It is a derivative of 1,8-dihydroxyanthraquinone. Reduction and degradation of the
chrysamminic acid gives the 1,8-dihydroxyanthraquinone.
The nitro grounds are highly reactive and easily substituted by other groups. I didn't try making though aloes resin is easily obtained from the
internet. Mind you so is 1,8-dihydroanthraquinone which is used as a laxative I believe.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Boffis  | Hi Philou, I came across this compound while trying to find out about another compound with a very similar "trivial" name "chrysanisic acid" that is
3,5-dinitro-4-aminobenzoic acid. Your compound can be prepared from brown Aloes resin by the action of nitric acid and this reaction is actually used
as a test for the active ingredient of the resin, barbaloin. It is a derivative of 1,8-dihydroxyanthraquinone. Reduction and degradation of the
chrysamminic acid gives the 1,8-dihydroxyanthraquinone.
The nitro grounds are highly reactive and easily substituted by other groups. I didn't try making though aloes resin is easily obtained from the
internet. Mind you so is 1,8-dihydroanthraquinone which is used as a laxative I believe. |
That is a good start   
Natural product, some use as medecine...
--> product should be easily available.
Thank you for those valuable informations.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
|