numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Synthesis of t-nitrosobutane (w/ pictures)
I was interested in this compound because it is described as a "deep blue liquid" which I found I had to witness, as there are few low weight organics
with such vivid colors. The preparation is very straightforward, and the dimer seems to be stable and can be stored. I ran this reaction on a 95mmol
scale based on t-Butylamine.
Another topic that mentions nitroso compounds, but I believe they were working with an entirely different reaction: link
Reagents:
-20% H2O2, diluted from 27% chlorine free pool shock
-Sodium tungstate, hydrate, used from bottle as is
-t-Butylamine, distilled from CaH2 the day before to a perfectly clear liquid
Procedure:
1eq t-butylamine is added to an equal volume of water, and 0.035eq Sodium tungstate is dissolved in this, a small amount stayed undissolved, but this
cleared up later on. This is chilled in an ice bath, and 2eq of Hydrogen peroxide as a 20% solution in water are dripped in over an hour, keeping the
temperature between 15-20oC. The first few drops only resulted in a cloudy solution. The solution was stirred for another hour without the ice bath,
and here is what I came back to.

The procedure states salt can be added to clear the "emulsion", but separation was already great, so I didn't bother. The blue liquid was separated,
washed with very dilute HCL, and dried with MgSO4. It should be noted that at this point the dimerization to the clear solid is
significant, and I would recommend working with haste.

This was transferred to a RBF with a stir bar and a boiling chip. It distills at about 60oC and the blue liquid started crystalizing halfway through.
If you do distill it,disassemble as soon as finished, otherwise the material will dimerize in the joints, freezing them. One of the quickest
distillations I've done, this is about 5 minutes in - and if you look, I got it mid drop! 
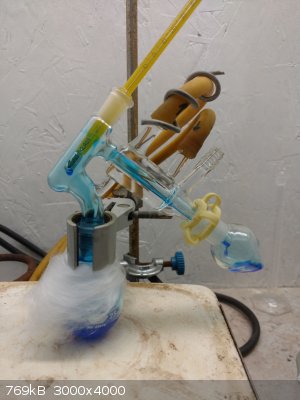
The product with it's dimer, I imagine it will all be dimerized by morning.
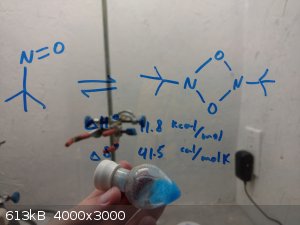
I will take it to the lab for NMR tomorrow, and let you guys know how pure it is.
This procedure was referenced from ACS Journal of Organic Chemistry, 1971, Volume 36, no.20
[Edited on 12-21-2016 by numos]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Very cool, nice write up.
Can you complex ions with this ring? Possible making a ferrocene like complex?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thats an amazing colour, thanks for sharing! Does the dimer dissociate on heating (without decomposition)?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That is a beautiful blue color, I rarely see nice colors in the lab, mostly colorless and brown. Copper makes some nice blue compounds, but very few
organics do.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Brilliant blue colour! Well done.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Superb numos !
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
That’s a spectacular synthesis. I love strong colour changes its gives me confidence in the reaction and that blue is very neat.
I want to try it. I will have to risk ordering some sodium nitrite even though it’s on the UK watch list.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
What for?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Tsjerk  | Very cool, nice write up.
Can you complex ions with this ring? Possible making a ferrocene like complex? |
Nitrite ion can act as a ligand; I wonder if this monomer would as well...
ETA: Nitrosoalkanes can act as ligands: http://pubs.acs.org/doi/abs/10.1021/ja00350a030?journalCode=...
[Edited on 21-12-2016 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Oops I was thinking about some of the other blue compounds like smurf blue. I don't have any tungstate.
But now I think about it, I have some spare TIG rods so diy sodium tungstate should be easy.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by wg48  | | But now I think about it, I have some spare TIG rods so diy sodium tungstate should be easy. |
Cool !
Please please show us how you do that.
Glad you said it 'should be easy' so i guess we'll see some of your awesome chemistry soon !
Can't wait to see how it is done 'cos i also have some tungsten welding rods in the shed.
Exciting times !
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I wonder if... this procedure would work for TRIS (trishydroxymethylaminomethane or 2-amino-2-hydroxymethyl-1,3-propanediol) (HO-CH2-)3C-NH2.
This compound is pretty OTC if made from nitromethane and formaldehyde polycondensation
CH3-NO2 + 3 CH2=O -base-> (HOCH2)3C-NO2
(HOCH2)3C-NO2 -reduction-> (HOCH2)3C-NH2
Then maybe
(HOCH2)3C-NH2 + H2O2 + Na Tungstate --> (HOCH2)3C-N=O
(HOCH2)3C-N=O + (HOCH2)3C-N=O --> (HOCH2)3C-N(O)=N(O)-C(CH2OH)3
or
(HOCH2)3C-N=O + H2N-C(CH2OH)3 --> (HOCH2)3C-N=N-C(CH2OH)3 + H2O
Upon reaction with HNO3 or N2O5 those two will:
1°) simply be oxydised back to the wel known explosive liquid nitroisobutylglycerol trinitrate ester (O2NO-CH2-)3C-NO2
2°) be nitrated to potentially novel energetic materials
(HOCH2)3C-N(O)=N(O)-C(CH2OH)3 + 6 HNO3 --> (O2NOCH2)3C-N(O)=N(O)-C(CH2ONO2)3 (A blue explosive  ?) ?)
(HOCH2)3C-N=N-C(CH2OH)3 + 6 HNO3 --> (O2NOCH2)3C-N=N-C(CH2ONO2)3
Those two would likely be solid and more powerfull than PETN (pentaerythritol tetranitrate = C(-CH2ONO2)4)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
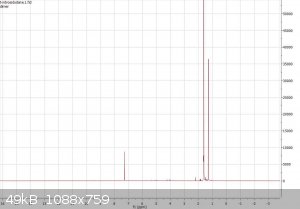
I ran an NMR (1H, CDCl3) and it's quite interesting. The right most peak seems to be from the dimer. The second right most I believe is the
water peak, but it's abnormally large (integration ratios of three main peaks, from left to right: 0.24@7.26ppm, 3.25@1.58ppm, 1@1.26ppm) The spectrum provided by Sigma for the dimer also has a large water peak. Anyone have any idea of what causes this? Chemdraw predicts a sole peak at
1.22ppm which corresponds to the right most one... But I still am at a loss for why the 1.58ppm peak is so large... there's no way there was that much
water in the sample.
Tsjerk: I hadn't thought of that, I'll get back to you if I discover anything experimentally.
DJF90: so I know that dissolving the dimer in solvent causes it to revert to the monomer, the nmr tube was clear when I ran it, but was blue, maybe
half an hour later when I checked back. The paper does mention that the monomer is favored at higher temperatures, and that redistilling the dimer
will yield t-nitrobutane. I can try heating some later and see what happens.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Congratulations on the nice synthesis.
The two singlets are probably the two different t-Bu groups, one from the monomer and the other from the dimer. You can see if their ratio changes by
taking the 1H NMR again a day later. If you pay attention to the 13C NMR from Sigma, you will notice there are also two sets of signals. Water is
probably a small peak partially overlapping with the one at 1.58 ppm (the picture cannot be zoomed that well, but it appears there). There might be
hidden somewhere also a tiny peak of the cis-dimer, if present at all.
By the way, I don't think you got the molecular structure of the dimer correctly. A 4-membered all-single bond cycle is unlikely. At the first glance
it appears to have a higher energy than the monomer. Nitroso compounds generally dimerize by the formation of -NO=NO- groups. See the structure from
XRD as reported in J. Chem. Soc., Perkin Trans. 2, 1988, 701-703 (DOI: 10.1039/P29880000701).
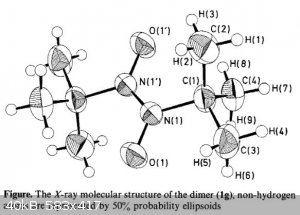
Attachment: Structure of the trans-Dimer of 2-Methyl-2-nitrosopropane.pdf (337kB)
This file has been downloaded 609 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
The two singlets are probably the two different t-Bu groups, one from the monomer and the other from the dimer. You can see if their ratio changes by
taking the 1H NMR again a day later. |
I agree completely, especially given the following:
Quote: Originally posted by numos  |
...the nmr tube was clear when I ran it, but was blue, maybe half an hour later when I checked back. |
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Incidentally, : cool prep. Where did you get t butylamine?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by numos  | I ran an NMR (1H, CDCl3) and it's quite interesting. The right most peak seems to be from the dimer. The second right most I believe is the
water peak, but it's abnormally large (integration ratios of three main peaks, from left to right: 0.24@7.26ppm, 3.25@1.58ppm, 1@1.26ppm) The spectrum provided by Sigma for the dimer also has a large water peak. Anyone have any idea of what causes this? Chemdraw predicts a sole peak at
1.22ppm which corresponds to the right most one... But I still am at a loss for why the 1.58ppm peak is so large... there's no way there was that much
water in the sample.
Tsjerk: I hadn't thought of that, I'll get back to you if I discover anything experimentally.
DJF90: so I know that dissolving the dimer in solvent causes it to revert to the monomer, the nmr tube was clear when I ran it, but was blue, maybe
half an hour later when I checked back. The paper does mention that the monomer is favored at higher temperatures, and that redistilling the dimer
will yield t-nitrobutane. I can try heating some later and see what happens.
|
Following the diazo di N oxyde structure
(CH3)3C-N=O + O=N-C(CH3)3 <==> (CH3)3C-N(O)=N(O)-C(CH3)3
And as exposed into Nicodem's post picture of the trans dimer conformation, one may see that each oxygen atom onto the nitrogens comes into close
vicinity to one or two hydrogen atoms from a methyl group --> 2 or 4 interractions O free doublets and H atom...might this explain you HNMR spectra
with large H-O peak?
Here also I wonder,
If by heating/distilling (CH3)3C-N=O you get oxydation to (CH3)3C-NO2...then what would the other molecule entering the disproportionation (CH3)3C-N=O
be reduced into?
--> Azo t-butane (CH3)3C-N=N-C(CH3)3?
--> hexamethyl-ethane (2,2,3,3-tetramethyl-butane) (CH3)3C-C(CH3)3 and N2(g)?
This would also be interesting for the nitroso-TRIS and dimer I was wondering about a few post ago.
Or do you mean that O2 from air is oxydizing the nitrosocompound/dimer to the nitro compound?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
I think you are correct, I saw that square structure somewhere originally, but I can't for the life of me remember where, even the paper I referenced
has the R-ON=NO-R structure... Sorry about that.
Quote: Originally posted by PHILOU Zrealone  |
Here also I wonder,
If by heating/distilling (CH3)3C-N=O you get oxydation to (CH3)3C-NO2...then what would the other molecule entering the disproportionation (CH3)3C-N=O
be reduced into?
--> Azo t-butane (CH3)3C-N=N-C(CH3)3?
--> hexamethyl-ethane (2,2,3,3-tetramethyl-butane) (CH3)3C-C(CH3)3 and N2(g)?
This would also be interesting for the nitroso-TRIS and dimer I was wondering about a few post ago.
Or do you mean that O2 from air is oxydizing the nitrosocompound/dimer to the nitro compound? |
When I did the distillation, there was originally only the dark blue monomer in the distilling flask, by the end of it, there was a clear liquid with
a greenish tint in the flask with a much higher boiling point. I imagine some of this oxidation was taking place there. I'm speculating but I do
believe it is just air oxidizing it. The paper isn't very clear on the actual oxidation, Tell ya what, I'm busy until after Christmas, but I'll run
two distillations with the stuff, one under Argon, if oxidation occurs in that run then you might be correct and I'd be interested in making a larger
batch and running more tests. I want to steer clear or energetic materials because they become difficult to store/dispose of safely, if you have the
means to safely try this with the Tris(hydroxymethyl)aminomethane, I would love to see it!
Also, unionised, this was an old bottle from sigma, the liquid was black before I distilled it. There's also a ton of amines at my work, but that
defeats the purpose of home chemistry... although I suppose nmr does too 
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Very nice, numos!! Always nice to read about some more chemistry - I also kind of like nitroso compounds (and S-nitrosothiols!) so it's nice to read
about the weirder nitrosoalkanes (vs nitrosoaromatics)!
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Nice NMR, I agree that the two main peaks are the monomer and dimer, most likely, the peak at 7.26 is residual CHCl3. I still love the blue
distillation, almost as colorful as a bromination, but looks like you are distilling a Smurf.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I tried this synthesis and here is my report.
I dissolved 0,57 g of Na2WO4.2H2O in 5 ml of water and added 5 ml of t-BuNH2. I also prepared 30 ml of 20% H2O2 and 1+10 HCl for washing. Everything
was cooled in the fride before starting the reaction. t-BuNH2 solution was stirred in a cold EtOH bath and from seperatory funnel 20% H2O2 was slowly
dripping in to the reaction mixture. Reaction mixture quickly turned green a later blue. Blue organic layer started to form pretty quickly. I let
reaction run for 2 hours and than put everything in to a separatory funnel. I discarded aqueous layer and washed organic layer with HCl. After that
organic layer turned in to weird blue gel. I shake it with some water and compound turned in to the blue liquid again. I dried organic layer with
anhydrous Na2SO4 and filtered it in to the clean vial. I didn't measured yield, but it obviously isn't great. I'll repeat this experiment again, this
time on bigger scale.
[Edited on 12-6-2023 by Bedlasky]
  
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Bedlasky well done! Nice color!
I found that another compound nitrosobenzene should be green liquid which solidifies to white solid:
http://www.orgsyn.org/demo.aspx?prep=CV3P0668
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
I just grazed over the thread, but no one started from sodium azide for that?
|
|
|