bis pyridine dichloride Cobalt II synthesis
Cobalt has raised huge attention as a possible alternative to rare earth based organometallic catalysts and this make it worth enough for some
chemists to do a lot of research on Cobalt catalysts.
I am gonna more show the practical procedure and if anybody has any theory questions you can ask me everything .
Experimental procedure :
In a 100 ml round bottom flask we put
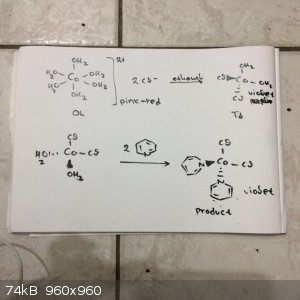
0,01 mol [Co(H2O)6]Cl2 that has reddish-pink colour m = 2,38 g
and 30 ml of ethanol
with heating and stirring the cobalt II chloride slowly dissolves and the solution takes straight a violet purple-blue colour. This means that the
geometry of the molecule change from octahedral to tetrahedral
after we make second solution of 2 ml of pyridine (0,025 mol) in 5 ml of ethanol and we put this in the first solution we see that the colour not
change (violet-purple-blue colour)
After 10 seconds a solid start to precipitate
The colour changes from pink to purple indicating that the geometry of the cobalt two complex change from octahedral (bigger 10 Dq ->pink-rose-red
colour) to tetrahedral (smaller 10Dq ->violet-blue colour )
This happens since the 10Dq of tetrahedral is 4/9 of the 10 Dq of octahedral complexes in general.
This all time classic synthesis show us how a change in geometry has a dramatic effect on the colour of the coordination compounds.
Last step after filtering we wash the crystals first with one portion of ethanol to wash away any extra pyridine . Let the crystals dry in air and
dispose the pyridine containing waste properly !!!
So our crystal in the end is kind of rose indicating octahedral geometry . But our compound in many solvents show a blue purple colour indicating
tetrahedral geometry in the solvents (for example DCM) . So this happens because our compound when it is solvated it make particles of size of a
molecule only and this has tetrahedral geometry and so a blue pruple colour.From the other side the crystal is a polymer of course and has a pinkish
rose colour witch indicates octahedral geometry. This happens because in the crystal polymer the tetrahedral changes form and the chlorines become
bridges joining two cobalts together forming a poly pseudo octahedral crystal structure that has a pinkish colour. To become more understandable what
I am saying the last picture I uploaded show the structure of the coordination polymer .
Reaction mechanisms (Sorry for handwriting and not using chemdraw and avogadro... )
[Edited on 20-1-2017 by Zool]
[Edited on 20-1-2017 by Zool]
[Edited on 20-1-2017 by Zool]
![reaction mixture after reaction [Co(Cl)2(py)2].jpg - 85kB](https://www.sciencemadness.org/whisper/files.php?pid=472403&aid=56362)
[Edited on 20-1-2017 by Zool]
 
[Edited on 21-1-2017 by Zool]

[Edited on 21-1-2017 by Zool]
[Edited on 21-1-2017 by Zool]
[Edited on 21-1-2017 by Zool]
![crystal structure of [Co (py)2 (Cl)2].jpg - 75kB](https://www.sciencemadness.org/whisper/files.php?pid=472403&aid=56378)
[Edited on 21-1-2017 by Zool]
|