Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
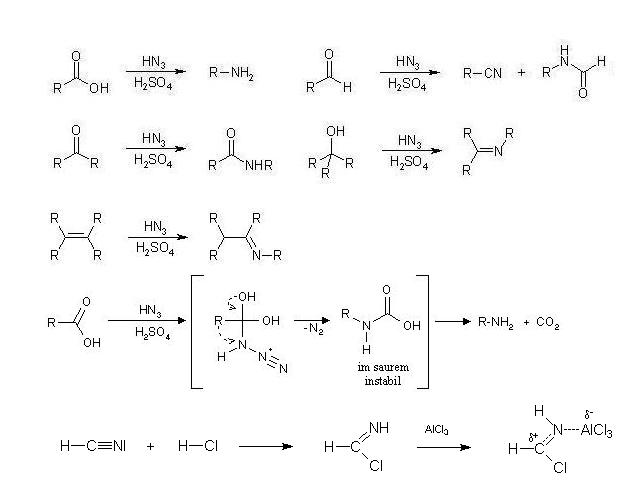
N-Substituted Amines
Today i came up with a some nice suggestions to the amin
manufacturing by some simple reactions with ammonia or
ammonium chloride and some substituents. Have anyone
some infos to the process within propanol, butanol
andpentanol and if this can work with H2SO4 when a
mixture of concentated sulfuric acid and f.i. propanol is
heated with some portions of ammonium choride ?
CH3-CH2-CH2-OH + H2SO4 + NH4Cl (NH3) -> CH3-CH2-CH2-NH2
I think the following compounds are useful to make some interested amines:
methylylamin (methylamin hydrochloride by the way HCl)
from methanol ethylamin (ethylamin hydrochloride by the way of HCl) from ethanol.
1-propylamin from 1-propanol
1-butylamin from 1-butanol
1-pentylamin from 1-pentanol
Seems interested, maybe methylamin or ethylamin can be
prepared by the way of anyhdrous MeOH or EtOH by boiling
with ammonium chloride in concentrated H2SO4. Some infos
may help, maybe it gives trimethylamin with the correct
rections conditions.
CH3-CH2-OH + NH4Cl + H2SO4 -> CH3-CH2-NH2 (CH3)3N
I would mean it is possible to make the amines from carboxylic acids with a similar process.
The Schmidt reaction told a amin can be obtained from a
caboxylic acid by the way with cyanides (Gatterman- Koch)
and n-substituted carbamides.
Maight this is correct that a modifyed Gatterman-Koch synthesis in precence of a cyanide will give a n-substituted
amin by the way of the intermediate cyanonitrogen ion.
Seems something wasteful but the simple synthesis should
work to obtain a amin, a amide or a secondary amin.
CH3-CH2-COOH + H-CNI -> CH3-CH2-C(OH)-N-NNI-> CH3-CH2-NH2
CH3-CH2-COOH + H-CNI -> CH3-CH2-C(OH)-N-NNI-> CH3-CH=N-OH
hydroxylamin from formic acid
methylamin from acetic acid
ethylamin from propanoic acid
1-propylamin from butyric acid
1-butylamin from pentanoic acid
Some infos to my issues will help and i hope i can found something more in the next days.
[Edited on 11-12-2006 by Mason_Grand_ANNdrews]
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Whats to stop the sulfuric acid with reacting with the ammonia? The ammonium ion can't be a nucleophile.
What you need to do is heat the alcohol with H2SO4 and a halide salt until you get R-X. Then you treat that with ammonia.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
You have shown the initial reactions of the carboxylic acid and alkene substrates as being, not with NH3 as the text of your post suggests, but with
HN3 instead, which is hydrazoic acid, a highly poisonous and explosive substance. In view of the mechanism you give in the third equation, showing the
reactive intermediate with an azo group, is hydrazoic acid the reagent that you intended/
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I have some useful issues to my last post to the amin
manufacturing with H2SO4 and ammonium chloride at the
moment. I would advice that the "Leuckard-Wallach
reaction" to make a amin or secondary amin by the way
of a alkylated amin with formic acid a reductant and
formaldehyde as a carbonyl compound or reductant is useful
with MeOH only.I have designed a little synthesis to produce
methylamin hydrochloride and trimethylamin hydrochloride
with the common synthesis.
Methylamin Hydrochloride (Methylammonium Chloride):
Methylamin hydrochloride (mp 228 - 231 °C) is obtained by
heating a formaldehyde solution with ammonium chloride at
around 105 °C.
2 H2C=O*H2O + NH4Cl -> CH3-NH2*HCl + HCOOH + 2 H2O
Maigth the process will work by adding a chilled mixture of
ammonia solution and HCl. (155 mL 25% ammona solution
per 131 mL 25% HCl). Prepare a mixture of 256 mL 35%
formaldehyde solution and 53,5 g of ammonium chloride in a
1000 mL flask, seal the flask with glass wool and set the
flask to a hotplate or a stirrer. At next, slowly heat up the
flask to 105 °C and continue heating for one hour at this
temperature. Let cool the mix, pour the content of the flask
into a 500 mL beaker and heat the mixture above 105 °C to
vaporize the formic acid HCl and water and to destroy the
remainders of formaldehyde. The temperature should stay
below 228 °C, the product should no decompose. The dryed
mass of methylamin hydrochloride is then collected
and is ready to use. Yield should be around 27 - 33 g, 45 -
50%.
Trimethylamin Hydrochloride (Trimethylammonium Chloride):
The synthesis of trimethylamin hydrochloride (mp 273 - 278 °
C) is similar to the methylamin hydrochloride process but the
temperature of reflux is different and the reductant is
paraldehyde.
Reflux 268 mL fresh distilled paraldehyde (bp around 124 °C)
for four hours with 53,5 g of ammonium chloride at around
160 °C, then vaporize the remainders of side products below
273 °C and colled the mass of trimethylamin hydrochloride.
Yield should be minimum 38 g.
I dont know if it correct, MeOH decompose when it heated
strongly in concentrated H2SO4 at the thermal
decomposition and NH4Cl too. It should give methylamin
hydrochloride, trimethylamin hydrochloride and besides a
little quantity of di/trichloromethane.
CH3-OH + H2SO4 + NH4Cl -> CH3NH2*HCl + (CH3)3N*HCl + CH2Cl2 + CHCl3 + NH3 + HCl + H2O
Have anyone some infos to the thermal decomposition of
MeOH ? I dont know if the process will produce
nitrogen trichloride (NCl3) and the mix explodes.
Maybe someone will test a small batch. A correct calculated
procedure will have a good chance to produce the product
what you will have. Might that will work:
Trimethylamin Hydrochloride Snthesis from H2SO4/MeOH/NH4Cl (Combined Snthesis):
Prepare a mixture of 500 mL of 99% sulfuric acid and 27 mL
anhydrous MeOH in a large flask and set the flask for reflux
and add 1 g of ammonium chloride to the mixture. At next,
heat the flask to about 100 °C and add in small portions 110
g (107 g calculated) of NH4Cl to the mixture by chilling and
heating it, wait for react and reflux the mixture for additional
one hour at 100 °C. Let cool the flask, remove the flask from
the distillation equipment and the little amounts of dilute
di/trichloromethane can be separated by distillation. Pour
the content of the flask over a filter to collect the
trimethylamin hydrochloride, wash it with several portions of
water and dry it in a oven. Have some a useful idea to clean
the product from the remainder of H2SO4 by dissolving it in
water ? I would mean a quantity of around 1 mol of
ammounium chloride per 1 mol of MeOH in 100 mL 95-98%
H2SO4 will give predominant methylamin hydrochloride.
I need some time to convert some useful procedures to the schmidt reaction, may it can help.
[Edited on 15-12-2006 by Mason_Grand_ANNdrews]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Would a mod please take the time to move this thread to Detritus? All this bulshitting is an offence to the science of organic chemistry. I dare not
to think of the intellectual damage a beginner would suffer if taking any of this seriously. 
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Excuse me guys that i suggested this procedures. I don`t
have every day a detailed synthesis wich is useful. I think
this is not a reason to move the thread to Detritus. The
ammonium ion can be a nucleophile or a intermediate ion but
is it right with a somewhat dangerous process within
hydrazioc acid ? I know hydrazioc acid is formed by heating a
mixture of sodium azide in dilute NaOH solution in a small
quantity less than 5%.
hydrazoic acid
HO-CH2-CH2-CH2-CH3 + NaOH(aqu.) + NaN3 ->
intm. N=N-N-CH3-CH2-CH2-CH2-OH ->
H2N--CH3-CH2-CH2-CH2-OH
Migth this give the amin when dangerous side products are
removed by a careful disttillation. I suppose there are many
possibilities to produce some of the amines. I'm sure i have
more what is much better to some of mentioned
syntheses.
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | I know hydrazioc acid is formed by heating a
mixture of sodium azide in dilute NaOH solution in a small
quantity less than 5%. |
Even wikipedia says you have to acidify. Hydrazoic acid is a weak acid; if you want to make HN3 from sodium azide, it's best to use a solution of an
acid stronger than HN3 (depending on the purpose). For instance, concentrated sulfuric acid is sometimes uses in the Schmidt rearrangement. Whatever be the case, I suggest you think it over whether you want to do experiments with HN3 or not. With your background,
I'd suggest you don't. Hydrazoic acid has a toxicity similar to HCN. It's OK as long as you have enough common sense to use it, but I wouldn't
recommend it to the novice.
[Edited on 25-12-2006 by Sergei_Eisenstein]
damnant quod non intelligunt
|
|
|
Brie
Harmless

Posts: 13
Registered: 3-6-2006
Member Is Offline
Mood: No Mood
|
|
neucleophile=electron donor
amonnium ion=positively charged ion with no damn electrons to share
|
|
|