Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Salt containing pentazolate ion isolated
I thought some people here might be interested to know. It's pretty stable, and the formula is
(N5)6(H3O)3(NH4)4Cl
Source:
http://science.sciencemag.org/content/355/6323/374
[Edited on 1-31-2017 by Metacelsus]
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
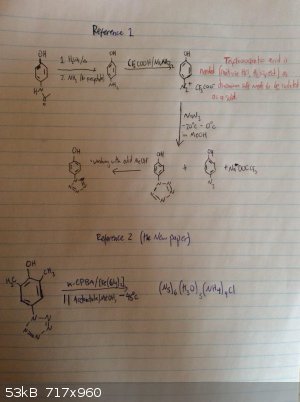
Ok I love this. The pentazole ring has been formed before, it is stabilized by being attached to a benzene ring. In fact, now that I look into it, a
somewhat stable pentazole compound can be made from paracetamol in three steps, which I would be doing now if it didn't require the use of
trifluoroacetic acid. But still, that's not a very obscure reagent and most professional labs will have that, but it's not a thing amateur labs have.
This new paper manages for the first time to cleave the benzene ring - pentazole bond. This is amazing, especially considering m-CPBA is not that
crazy of a reagent (once again, no home lab will have it but for what i've seen it's a well known lab chemical.) So the thing that makes the C-N bond
cleavage, according to the new paper is "formation of cyclo-N5ˉ from arylpentazoles proceeded more efficiently upon increasing the number of
electron-donating groups at the meta/para-position of the aryl groups". So the reaction probably doesn't work with the top pentazole, which is a
shame. Because that would be paracetamol to free pentazole in a handful of steps.
Still, this is the new forefront of energetic materials research, and I really don't think it's out of reach for us at home.
Reference one: http://sci-hub.bz/10.1021/jo0110754
Reference two (new paper): http://sci-hub.bz/10.1126/science.aah3840
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
It would be interesting to know what the density of the salt is. It looks like it might be a decent energetic on its own (81% nitrogen), though it is
unfortunate that the chloride ion cannot be replaced with nitrate (but maybe perchlorate was worth a try?). Of course, slow decomposition at ambient
temperature precludes its practical use, but very promising news nonetheless.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I highly doubt an oxidizing anion would be able to be anywhere near a pentazole ring without causing it to blow.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
This is so fucking sexy.
Quote: Originally posted by zts16  | | I highly doubt an oxidizing anion would be able to be anywhere near a pentazole ring without causing it to blow. |
Under non-hot conditions nitrate and perchlorate can barely be counted as oxidizers. Other highly-oxidizable species can easily form those salts like
TAG or hydrazine. The lack of the stability in their attempts to prepare those salts likely lies in crystal structure H-bonding being removed
compared to the ammonium/hydronium mixed salt.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Maybe that iron or other metals (Co, Cr, Ni, Mn, V, U, ...) may form ferrocene (or metallocene) like structures
--> pentaz(a)-ocenes Me(N5)2 
Wich prove to be stabler  ? ?
Or an explosive disaster  (owing to metallic core catalytic effect) ? (owing to metallic core catalytic effect) ?
FeCl2 + N5(-) -THF-> (N5)Fe(N5) + 2 Cl(-)
Yeah FeN10 --> Fe + 5 N2(g) + a lot of energy and heat.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
While we are on the subject of esoteric energetics, did anyone see this:
http://www.telegraph.co.uk/science/2017/01/27/us-scientists-...
There is still some doubt as to whether it actually was metallic hydrogen that they made, and if it was, if it will be metastable at STP. But if it
was, and if it is, the predicted performance is quite extraordinary.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
That is definately interesting stuff,
I really want to believe it true, however Nature dosnt think so.
http://www.nature.com/news/physicists-doubt-bold-report-of-m...
|
|
|