JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Elementary Work on Schiff Bases
I finally got started working on schiff bases at the university so I wanted to keep tabs of all the work I do on here for quick reference when writing
it all up at the end of the project. If this is inappropriate I understand the mods are free to detritus the topic. I also thought some people might
enjoy following along and the occasional pictures.
Preparation of N-Benzylideneaniline:
To begin work on studying the properties of schiff bases, otherwise known as imines, a relatively stable substrate is needed. Aromatic schiff bases
are generally more stable than the purely aliphatic counterparts although the nature of those will be looked into as well.
The procedure is adapted from L.A. Bigelow and H. Eatnough, Org Syn., 8, 22 (1928)
Materials:
-Benzaldehyde (Sigma Aldrich)
-Aniline (Sigma Aldrich)
-95% EtOH (sourced from the school, not sure brand)
Methods:
Two 50 mL beakers were pre-weighed and set aside. Into one beaker was measured out approximately 10mL (9.943g) of aniline using a graduated cylinder.
Into the other beaker was poured approximately 11mL (11.068g) of benzaldehyde, also measured out using a graduated cylinder. A thermometer was then
placed into a 150mL beaker and the benzaldehyde was added along with a stir bar. With slight stirring, the aniline was poured into the benzaldehyde.
Immediately the temperature was observed to increase from 21C to a max of 45C. Attached is a photo of the reaction mixture. The mixture was left to
react for 15 minutes. During this time, 20mL of EtOH was added to a separate 150mL beaker. When the 15 minutes had been reached, the reaction mixture
was poured into the EtOH. After cooling the beaker with cold water the mixture was filtered. However the EtOH was not mixed well with the reaction
mixture so the crystals crashed out after passing through the filter paper. The slurry was taken out of the filtering flask and then re-filtered to
obtain the crystals. These were washed using a sparing amount of 95%EtOH. The crystals were then dried on a vacuum in open air for 10 minutes and then
collected. Weighing the crystals resulted in only 7.603g. of slightly yellow crystals. This represents only a 40.46% yield. The literature claims
yields usually hovering around 85-87%.
Notes:
The low yield can be accounted for by mechanical losses when trying to retrieve the slurry of crystals. The procedure also claims obtaining
approximately an extra 10g from concentrating the mother liquor. Tomorrow I plan on attempting to recover more product from the mother liquor as well
as characterizing the product through a melting point, running an H1-NMR, as well as preparing another schiff base substrate using acetophenone and
aniline.
Any thoughts?

|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Heating the reaction after mixing, pouring the reaction very slowly into the ethanol, and letting it sit overnight in a fridge/freezer before
filtering are all possible ways to up the yield.
You will find the reaction between ketones and amines much slower, it may not happen at all without heating, reflux in some solvent with a Dean-Stark
trap, sieves, or a chemical dehydrating agent. If you can get any decent yield with acetophenone, you are doing well.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
as an amateur dabbler in chemistry, I think that it is great that you share your progress with us.
later you could even produce a condensed version in prepublications.
The only problem that I forsee is - quoting SM as a reference 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Continued N-Benzylidenaniline Work-up:
After leaving the crystals to dry overnight the mass went down as excess EtOH left on the crystals evaporated. The final product weighed 6.923g
corresponding to a 36.84% yield. The mother liquor was put on a hot plate for 2 hours to try and concentrate the solution to saturation but no
crystals were observed precipitating upon cooling. However this was saved for future recovery attempts. A melting point was taken using a Mel-Temp
Apparatus and was 37-43C. This is below the literature cited values between 42 and 56C (the product is polymorphous). An H1-NMR in deuterated
chloroform was also taken and I'll attach the spectra. There is an aldehyde peak around 10ppm attributed to un-reacted benzaldehyde and a broad bump
around 6ppm attributed to un-reacted aniline. This spectra along with the melting point warrants a recrystallization.
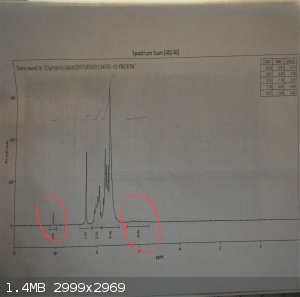 
[Edited on 2-3-2017 by JnPS]
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Preparation of Benzophenone Anil
Unlike their aldimine counterparts, ketimines are much harder to produce. Ketones are less prone to imine condensation due to steric hinderance and a
less electrophilic carbonyl carbon. To overcome this, procedures usually call for either extended reflux times, azeotropic removal of water from the
reaction mixture, or the use of a catalyst. The procedure adapted here, cited in the following post, calls for the use of a lewis acid catalyst.
Materials:
-Acetophenone (Sigma Aldrich)
-Aniline (Fisher)
-Zinc Chloride, anhydrous (Sigma Aldrich)
-Diethyl Ether (sourced form school, unbranded bottle)
Methods:
Two 10mL graduated cylinders were pre-weighed and set aside. A clean 100mL beaker was placed on a hot plate stirrer and primed with a stir bar and
thermometer. In separate graduated cylinders approximately 10mL of acetophenone (9.894g) and aniline (9.506g) was measured out. The acetophenone was
poured into the beaker and stirring was then turned on. As expected, upon the addition of the aniline no exotherm was observed. (see photos provided
in following post). The mixture was then heated on the hot plate with stirring to 160C. During this time the color changed from clear to a dark red
(see photos provided at various temperatures). This color change can either be due to the reaction proceeding or the aniline degrading. When the
mixture approached the 160C mark 1-2g of catalyst (1.550g of anhydrous zinc chloride) was weighed out. This was then freshly crushed with a glass stir
rod. Once the mixture hit 160C the catalyst was added in small spatula-fulls over the course of 1.85min. Upon addition the mixture immediately turned
a dark brown and began bubbling vigorously. The mixture was then held at 160C for 30min. During this time while attempting to maintain a constant
temperature through the hot plate controls the thermometer did read as low as 148C at one point but never lower. Eventually a system was discovered
consisting of alternating between increasing the hot plate dial between a range of 15 degrees every few minutes to keep the thermometer reading
between 158-162C. After the 30min were up the heat and stirring were turned off and the beaker was allowed to cool. Upon cooling the mixture
solidified into a black sludge. At this point the procedure called for the melt to be "boiled with a little benzene or ether". Unsure of why the ether
needed to be boiled, I decided to attempt purely extracting it with the ether to avoid the hazards of working with hot surfaces in the same hood as
ether. 20mL of diethyl ether was added to the black sludge. Upon addition some of the black goo dissolved and there was a crystalline precipitate
visible in suspension. The ether was then filtered and the beaker washed two more times with 20mL of ether. the washings were combined in one buchner
funnel and washed with an additional 5mL of ether. The crystals were then dried under vacuumed and transferred to a weighing boat. A total of 2.283g
of crude crystals were obtained representing a pitiful 14.43% yield. However due to the discoloration of the crystals, a recrystallization is needed
before further characterization of the product is carried out.
Notes:
-At the 9min mark the stir bar was deemed inefficient and switched out for a larger one. This probably did not affect the reaction but was noted for
scrutiny's sake.
-At the 19min mark a white "foam" was observed on the sides of the beaker which disappeared after cooling, unsure what this might be.
-To improve yields the mixture can be refluxed rather than heated in an open beaker as suggested by Dr.Bob and slower addition of the catalyst may
also be benefical. A longer reaction time may also prove worth pursuing when repeating this procedure.
-I'm in the process of using my N-benzylideneaniline to find a suitable recrystallization solvent but am open to suggestions if anyone has any ideas,
I suspect a suitable solvent for that imine should work for this one.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Procedure above adapted form: J.H. Billman and K.M. Tai, J. Org. Chem., 23, 535 (1958).
 
[Edited on 3-3-2017 by JnPS]

|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Further photos of Benzophenone Anil Prep
  
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Nice job, that is not an easy prep, I have had some hard times making imines from acetophenones, even with fancy glassware. It is a great way to make
alpha methyl benzylamines.
Often if you have a mixture of product and black tar, you can dissolve the product from the tar by washing the tar with ether (or hexanes, pet ether,
or pentane). It can dissolve the smaller molecular weight product from the polymeric crud and then if you do that multiple times you can often
extract a lot of reasonably pure product from some nasty black tars that way. I have had a few cases where with one recrsyatallization I can get
nice colorless crystals from a glob of what looks like road tar.
[Edited on 4-3-2017 by Dr.Bob]
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Workup of Previous Procedures
N-Benzylideneaniline Recrystallization
Although the crude N-Benzylideneaniline was reasonably pure, the melting point was still broad and the NMR showed trace, but detectable, amounts of
what is suspected to be unreacted benzaldehyde. Absolute ethanol was tested as a possible recrystallization solvent both to purify the crude product,
and verify that the solvent may be possible to work for recrystallizing the also crude benzophenone anil.
Materials
-2.42g N-Benzylideneaniline (prepared previously)
~20mL of Absolute EtOH (sourced from the school, unbranded bottle, not all used)
Methods
Approximately 20mL of absolute EtOH was poured into a 50mL beaker and heated to near boiling on a hot plate. In a weigh boat, 2.42g of the crude imine
product was weighed out. These crystals were then added to a clean 50mL beaker and placed on the same hot plate as the EtOH. A small amount of hot
EtOH was added to begin dissolving the crystals. Hot EtOH was added in small portions until all the crystals had dissolved, this was a total of about
10mL of EtOH. The solution was then taken off heat and allowed to cool. Once the beaker was at room temperature it was placed in an ice bath for 5
minutes to further cool the solution. After cooling in the ice bath no crystals were seen precipitating, to initiate crystallization a single small
crystal from the prepared crude product was added as a seed crystal. Immediately the crystals crashed out similar to the demonstrations done with
sodium acetate trihydrate. These were then filtered in a buchner funnel, dried on vacuum, and weighed. The total amount recovered was 1.538g
representing a 63.56% recovery. A melting point was then taken using a Mel-Temp apparatus yielding a melting point of 48-50C. The narrow melting point
indicates a higher purity product than before the recrystallization. However the NMR taken still indicated an aldehyde peak suggesting that some
benzaldehyde may still be trapped in the crystals. This can be due to the fast crystallization through the use of a seed crystal, although this is
speculative.
 
Recrystallization of Benzophenone Anil
Now that Absolute EtOH was observed to work as a suitable recrystallization solvent, an attempt was made to purify the crude crystals obtained from
the previous preparation.
Materials
-2.185g Benzophenone Anil (previously prepared)
~20mL of Absolute EtOH (sourced from school, unbranded bottle, not all used)
Methods
Like before, approximately 20mL of absolute EtOH was poured into a beaker and placed on a hot plate. The EtOH was again heated to near boiling. All
the dry Benzophenone Anil was weighed out, resulting in a total mass of 2.185g (the benzophenone weighed at the end of the initial prep must still
have been wet with some leftover EtOH). These crystals were then added to a clean beaker and placed on the same hot plate as the EtOH. After 10mL of
EtOH had been added, the crystals were not dissolving. There was the appearance of two layers, a mass of solids at the bottom and an upper layer of a
dark brown EtOH solution, supposedly containing some product. The EtOH layer was then decanted off. Unaware of whether the product was in the solution
or the undissolved solids, both beakers were taken off and allowed to cool. The EtOH solution was observed to have some crystalline solids
precipitating and the solids dried to form a solid mass at the bottom of the beaker. The EtOH layer was then cooled in an ice bath for 5 minutes to
precipitate more crystals. These were then filtered through a Hirsch funnel and dried on vacuum. Both these crystals and the un-dissolivng solids were
then characterized through taking a melting point with a Mel-Temp apparatus. However after reaching 150C the procedure was stopped due to lab time
constraints and this is already considerably above the literature value of 117C for benzophenone anil.
  
Notes
-The benzophenone anil product does not appear to be the desired product. A possible suspect is maybe the aniline chloride salt. Maybe upon addition
of the zinc chloride catalyst the aniline formed a salt rather than reacting with the benzaldehyde. The aniline chloride salt has a reported melting
point above 200C. In addition to the high melting point, the crystals would not dissolve in deuterated chloroform for taking an NMR. The inability to
dissolve in the chloroform and the high melting point make this a likely culprit, though honestly I am not sure what the crystals or undissolving
solids might be except that they are not the intended product.
-I'll be moving on to preparing a schiff base from an aliphatic amine and benzaldehyde. The lack of two aromatic rings should make characterization
through H1-NMR easier.
-Any suggestions of how to identify the solids or crystals from the benzophenone anil prep would be appreciated, next week I will try to take a
complete melting point to determine whether it is in fact the anilinium chloride.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Schiff bases with ortho substituion often act as interesting ligands. A good example is Salen, prepared from ethylenediamine and salicylaldehyde, and
its cobalt complex. Other interesting schiff bases in this class can be prepared from salicylaldehyde and 2-aminophenol, o-phenylenediamine and just
recently I successfully condensed this aldehyde with 4,5-diaminofurazan to produce beautiful orange red crystals of what appears to be the bis-schiff
base. A similar compound, used as a selective fluorescent reagent for magnesium, is the bis(salicylidene)-2,3-diaminoindole though this compound is
prepared indirectly. Many of these bases form brightly fluorescent complexes with aluminium magnesium zinc etc.
|
|
|