Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
How do you interpret NMR readings?
So I was watching NurdRage's videos a few days ago. One of the videos in his pyrimethamine series showed an NMR reading of the P-chlorobenzyl
acetonitrile that was made. He/she explained what each of the peaks on the NMR reading meant, and this got me thinking: How do you interpret the
meaning of what shows up on other NMR results?
Say for instance......various alcohols like methanol,ethanol,propanol and isopropanol,etc.
Edit: https://youtu.be/15ZkhhJcXXk?t=8m2s
Around the time the maker explains the NMR results for the video.
[Edited on 17-4-2017 by Volitox Ignis]
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Just google how to read NMR spectra, It's not terribly difficult. They usually teach it to undergrads in a first time Organic chem course. Just make
sure you specify which type of NMR. H1 NMR is the most common but C14 and Nitrogen NMR is also used at times. The technique of reading the spectra is
the same but you just have to know which atoms you're getting spectra on.
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spe... <- This link has some good info in case you have a hard time finding anything
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It's 13C. 14C has zero spin. Besides, it's radioactive.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
@Metacelsus Doh! *facepalm* Thanks for the correction, that's what I get for posting when I should have been sleeping
|
|
|
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Status update: I have started watching some videos about it a while ago. I am confident that if given the formula for a simple substance and its NMR
reading, I could probably guess the structure.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
We learn it in high school chemistry, C13 NMR is the easy one, wait until you see and try to decipher a proton NMR spectrum..
(This is for anyone else who stumbles across this thread and doesn't understand it)
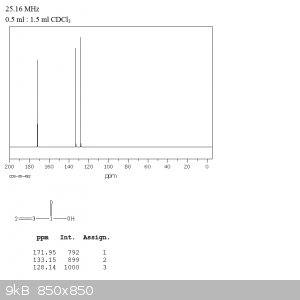
I've included a sample carbon NMR of propenoic acid as it contains a couple of groups. There are three peaks corresponding to the 'carbon
environments', which basically tells you how many carbons can 'see' different groups on adjacent carbon atoms. The X-axis displays the chemical shift,
which is the resonant frequency of a carbon atom compared to tetramethylsilane (TMS), used as an analytical standard for both C and H NMR - it tells
you what groups are present if they contain a carbon atom, like CH3, COOH, C=O, etc, and these groups all have a small designated range in the
chemical shift.
Deciphering the propenoic acid spectrum:
Acrylic acid has three carbon environments as I mentioned before, so let's break them down. One carbon on the molecule is within the carboxylate group
adjacent the double bonded carbons, this puts it in the range of 170-180, all the way on the left. The other two peaks correspond to the carbons on
either side of the double bond, being distinct because the carboxylate group slightly affects the magnetic resonance of the carbon atom it is bonded
to, increasing its shift value, but both are within the range of 110-140 as would be expected of a carbon involved with a standard double bond.
Figuring out the structure of a simple molecule isn't too difficult using this technique, although I find that aromatic compounds can throw some
people off because the benzene ring can create multiple, close packed peaks, especially when there are different substituents around the ring.
Image: http://www.chemicalbook.com/SpectrumEN_79-10-7_13CNMR.htm
|
|
|