RebeccaOlson
Harmless

Posts: 22
Registered: 9-3-2017
Member Is Offline
Mood: No Mood
|
|
Secondary amine to alkane?
Hey guys, I was wondering if there is any simple reaction to change a secondary amine to an alkane, for example N-Methylaniline to Ethylbenzene. Thank
you 

|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Simple reaction? Hell no
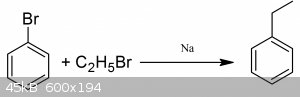
You could however demethylate to aniline and then diazatize the amine using NaNO2 and HCl at 0*C and then react that with the appropriate halide salt
to get halobenzene and then from there you could react that with haloethane and sodium metal to get ethylbenzene. The whole idea in general is kinda
impractical, it would be far easier to merely start from aniline.
http://www.prepchem.com/synthesis-of-chlorobenzene/
http://www.prepchem.com/synthesis-of-ethylbenzene/
The major issue is in the demethylation of aniline, i cannot find much info behond doing so biochemically but to be honest unless you are working from
a much larger molecule i would recommend scrapping the idea of working from N-methylaniline and just start at aniline.

|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
But there IS a simple reaction! You just need some thermal neutrons. Oh yeah, and your product is going to be super radioactive, and eventually decay back into what you started with, so don't even
THINK of using that ethylbenzene to make drugs.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|

Bravo Melgar, bravo.
|
|
|
laserlisa
Hazard to Self
 
Posts: 52
Registered: 5-2-2016
Member Is Offline
Mood: No Mood
|
|
That Wurtz-Fittig looks really cool. ( https://en.wikipedia.org/wiki/Wurtz%E2%80%93Fittig_reaction )
I would love to be able to make alkylbenzens without having to mess with dangerous organoilithium compounds.
But it seems surprisingly hard to find references of it being used in modern chemistry. Any obvious reasons for this? Harsh conditions and low yields?
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
@laserlisa,
here is a review including the Wurtz-Fittig reaction:
http://pubs.acs.org/doi/full/10.1021/om058054a
The reaction gives usually good yields if the cheaper halogen compound is used in excess. I think its usefulness is limited by the low functional
group compatibility. The sodio arenes exhibit a higher reactivity than their lithio counteparts.
[Edited on 23-5-2017 by Alice]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Oh, you're too kind! Though in all seriousness, that was not all that different from how they were first able to synthesize perbromates. Theory seemed to indicate that they could exist, it's just that nobody could figure out how to make them. Somehow they were
successful with the nuclear option before they'd tried using fluorine though; maybe they just had all these extra nuclear isotopes and needed to do
something with them before they decayed away?
If you actually want ethylbenzene, hydrogenation of styrene (available OTC as "resin thinner" from boating stores) is probably the most
straightforward way to go. You can even depolymerize polystyrene if you're desperate enough, though it's not pretty.
|
|
|
Nicodem
|
Thread Moved
22-5-2017 at 09:34 |