| Pages:
1
2
3 |
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
propionyl chloride
I am looking for a writeup on making propionyl chloride from propionic acid and thionyl chloride. I'll wing it from other acid chlorode writeups if I
have to but would like to use an established regimen if someone has it. All I know now is the ratio of agent to acid is 2:1 to avoid anhydride.
That would seem to mean putting them all together at the outset or adding the acid to the agent. A drying tube would seem appropriate. Not sure
about gas traps.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Vogels uses an example in which 0.5 mols are used as 47g !!??
(119g/mol x 0.5 mol = 59.5 g x ml/1.5 (20deg) = 39.7ml)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
First of all, thionyl chloride is not the only choice for this kind of transformation.
Secondly the use of excess reagent is for driving the equilibrium to the right, and is rtather wasteful of a hard to obtain reagent that is difficult
to prepare.
There are many threads here about other chlorinating reagents for making acyl chlorides and acyl anhydrides.
I'd suggest using the search engine.
Several of us have posted articles with general experimental details for more than one reagent, and details on how to prepare one of the most useful
reagents.
Finally, it seems I didn't need to tell you how to make propionic acid, or did you buy it?
Search on:
chlorination
acid chloride
anhydride
There are two or three reagents to find, apart from the ones Vogel cites. All of the ones he mentions are now difficult to obtain in many parts of the
world due to CWC restrictions. Those are thionyl chloride, phosphorus trichloride and phosphorus oxychloride. But just move on to the alternatives.
The alternatives are:
Oxalyl chloride
Cyanuric chloride (CC) also called TCT or trichloro-s-triazine
The first is rather expensive, but it can be made from the second.
The second is an inexpensive solid.
Do not confuse CC/TCT with trichloroisocyanuric acid which is closely related and also useful.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
What about sulfuryl chloride, from SO2 and Cl2? While it's used as a chlorinating agent, I remember seeing references to its use for making acid
chlorides. Or does it give too much in the way of side products?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Normallr if you want to go this route, per an old article cited on Rhodium, you use it to make anhydrides not acyl chlorides.
And you make sulfuryl halide in situ. So, sulfur and Br2 get you your reagent, anhydrous sodium acetate is added and a strange semisolid semiliquid
form, bromine color disappears. After distillation under reduced pressure Ac2O in high yield is redistiller through a fractionating column at
atmospheric pressure. It's a Russian prep Chemistry & Industry in the 40s IIRC.
While you could do that with chlorine it would be less convenient.
And I regard either as pretty inferior to the use of CC/TCT with or without DMF. Unless you have a lot of Br2 to spare and who does? I pay $100 a Kg
for mine I think CC is a lot cheaper than that.
Bromine and sulfuryl chloride are both dangerous and unpleasant to work with. CC is a solid, it's pungent but much milder than those.
You could also go the longer way round the block and make oxalyl chloride with CC, then use it to make either the acyl chloride or the anhydride as
you desire.
But better to save oxalyl chloride, which is just as nasty as Br2 to work with, for those special occasions when nothing else will do.
Like making POCl3 from P4O10 or (maybe) PCl3 from phosphorous acid (excess reagent dehydrating it to P2O3 in situ). Or AsCl3 from the oxide. Verboten
chemicals not easily accessible any other ay.
Oxalyl chloride can be purchased but it is not cheap. As CC is cheap and so is oxalic acid or anhydrous sodium oxalate, making it is more economical.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Br2 and S do not make sulfuryl bromide, but sulfur dibromide instead. I assume it was a typo.
Instead of sulfur and bromine, you can use sulfur dichloride or disulfur dichloride which are both cheap and easy (though NOT pleasant) to make by
chlorination of sulfur.
I did the preparation of Ac2O from Br2, sulfur and NaOAc. It didnt work well. The product was seriously impure as seen from the distillation curve,
and also had a funny smell probably from brominated derivatives.
$100 for 1 kg of bromine? What do you pay for a kg of NaBr or KBr? It would surely be cheaper to make it yourself.
I once paid 80€ for 1 Liter (not kg) of bromine (I have only a fraction of it left). That was not too expensive. But the prices have risen
considerably until now. I would have to calculate if it is cheaper to make than to buy it.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Yes I know what S and Br2 gives you on paper. I am reporting what the Russian piece cited on The Hive/Rhodium claimed was the mechanism. How they got
from S and Br2 to SO2Br2 is a mystery to me, and I have not tried this prep to see if it works, since I found far better ways to get to Acetyl
Chloride and Acetic Anhydride.
I'd guess either SBr2/S2Br2 oxidized readily or that these were the brominating agent and not sulfuryl bromide as claimed. Russian error, translation
error or Hive error? Dunno. I have it here somewhere in hardcopy and when I find it I'll scan and upload.
The most irritating thing about this prep was that it called for some Ac2O in the starting mixture. Now what is the point of requiring some product to
make the same product?
I found this complete archive of the several procedures including the one I had in mind on Erowid, attached bwlow. The error regarding drying temp has
not been removed. All reference to SO2X2 (X=Cl, Br) has been removed. Instead the active agent is given as suflur chloride or bromide. So either my
memory is bad or someone revised this. <shrug> But I specifically also recall mention of the stirred mix being a peculiar semisolid semiliquid
that seemed to slidify when stirring was interrupted then liquified again when stirring resumed. That vivid description stuck in my mind, but does not
appear in the text below, so I think on Rhodium there was more discussion of this procedure and maybe that's where the SO2Br2 mechanism was advanced.
------------
"Acetic Anhydride and Propionic Anhydride
Drone #342:
Here's what everyone's looking for. Some things are a little wierd about it, like the fact in the acetic anhydride synth they use a small quantity of
acetic anhydride as a solvent. However, as one sees in propionic anhydride, such a solvent may not be necessary.
From "Chemistry & Industry", 1945, p. 382; "LABORATORY METHOD FOR THE PREPARATION OF ORGANIC ACID ANHYDRIDES" by Jehuda Orshansky and Eliahu
Bograchov.
"...(1) Acetic anhydride. To 50 g. acetic anhydride in a round-bottomed flask of 1500 cc. capacity, placed in cold water, 440 g. of powdered sodium
acetate (dried by fusion at 320 C) and at the same time a solution of 22. g of sulfur in 320 g. bromine is added while stirring. The operation takes
about 30 minutes.
"The mixture is then stirred for a further 5 minutes, after which period the initially dark brownish-red colour has changed into pale yellow, and the
anhydride is distilled off from a water bath under reduced pressure. The crude anhydrie (310 g) is redistilled under normal pressure, and the fraction
boiling between 134-138 C is collected. Yield, 295 g. of 98% purity = 87.5%. The so purified anhydride contains neither bromine nor sulphur compounds
and leaves no residue on evaporation..."
"(2) Propionic anhydride. To 40 g. fused and powdered sodium propionate in a flask of 250 cc. capacity a solution of 2 g. sulphur in 22 g. bromine was
added while stirring. The temperature was kept at about 50 C. When the operation was completed, the anhydride was distilled off in vacuo. The crude
product (25g.) was fractioned under normal pressure, and the fraction 155-156 C was collected. Yield, 23 g. propionic anhdride of 90% purity = 85%..."
The paper mentions that other alkali metals and alkali earth metals work just fine. Calcium propionate is a food preservative added to cheap white
bread to keep it from molding. With these nuggets of information, the most closely watched reagent on the DEA's watched list, propionic anhydride,
just turned OTC. I can almost see the fentanyl analogs clogging the opioid market already.
The one reason, and justafiably so, they poo-poo using chlorine (which does indeed work nicely) is that its a hassle to work with, especially
considering the fact that they'd be adding a gas to a solid to make a liquid. I propose that perhaps with the use of chloroform as a solvent, chlorine
could be bubbled in readily, and the reaction would go as previously stated. I assume they tried to avoid using extra solvents in hopes of staying
away from azeotropes messing up their products' purity, so this may or may not work, depending on what you're trying to do."
----------------
Bromine: Actually I paid $75 for 1.5 Kg (500 ml). Too many middlemen. Now that I have found the local agent for Carlo Erba I can probably get it for
$50. I think that is 33 cents a gram. They limit me to a couple bottles a month, but since they shouldn't be selling it at all (it's a MOD-restricted
item for which I should need a license) I don't complain as I doubt my requirements will ever get near 36 Kg a year.
I do use NaBr to replace HBr aq in bromination of some alcohols/glycols.
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
Attachment: http___www.erowid.org_archive_rhodium_chemistry_anhydrides.pdf (34kB)
This file has been downloaded 2616 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Oh, and @chemrox:
Vogel teaches that the SOCl2 methos cannot be applied to acetic and propionic acids because the bp's of the resulting chlorides are too close to that
of the excess SOCl2 to permit seperation by fractionation.
He advises that thionyl chloride be used for acid chloriced of normal C4 acids and higher.
So, take my advice and use CC instead.
Now, if you can't buy propionic acid or a propioniate salt, and elect not to oxidize 1-propanol for some reason, all is not lost.
Buy a opropionate ester like methyl or ethyl propionate and saponify it.
Often the esters of even watched acids are readily available (cf., phenylacetate esters, anthanilate esters).
But cheapest/easiest ought to be oxidation of n-propanol and I'd expect a wide variety of oxiders to work. See Vogel and take your pick.
[Edited on 8-2-2007 by Sauron]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Thanks one and all!
What a thread! you'd think all the uses of this common reagent were suspect! I'm working with fuels! Anyway, what I got, also from Vogels, is the
two have close boiling points but excess SOCl2 is destroyed with formic acid which can be separated by fractional distillation. I already have the
SOCl2. And as someone pointed out, the examples in Vogels do not use a great excess. Also, less environmental concern and fewer side reax. But what
a wealth of information this generated.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
What environmental concerns might there be with CC? The byproduct is solid cyanuric acid, and that is useful in and of itself (you can chlorinate it
to trichloroisocyanuric acid for example.)
SOCl2 generates SO2 (as in acid rain) and HCl while oxall chloride, CO and CO2 (greenhouse gases) but I would say the two are about equal on that
score.
However since you already have the stuff, do as you please. I suspect that if you employed a H-scavenger like Pyr or TEA, you would not need any
excess of SOCl2 at all, and the resulting amine hydrochloride would be solid amd insoluble in your rxn mix, just filter off and wash. The point of the
excess reagent was to drive rxn to the right against competition from HCl being formed. The H-scavenger would do same thing, snap up the acid as it is
made. The SO2 will not trouble you, it will offgas.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
A Convenient Preparation of Volatile Acid Chlorides
HERBERTC . BROWN
Journal of the American Chemical Society 1938, vol:60 iss:6 pg:1325
Summary
A simple general procedure for the preparation of volatile acid clorides is described, involving the distillation of the desired acid chloride from a
mixture of benzoyl chloride and an organic acid. The procedure has been used in the prepara- tion of twelve aliphatic acid chlorides with yield of
70-90%. The mechanism of the reaction is discussed. NO definite condusions concerning the mechanism could be reached.
Attachment: A Convenient Preparation of Volatile Acid Chlorides .pdf (439kB)
This file has been downloaded 3471 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks @solo. Another tool in the arsenal.
Time for a cost analysis and comparative study of the facility of this vs TCT vs oxalyl chloride and for those who can still get it, thionyl chloride.
The last is not an option for me.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Benzoyl chloride is a way better choice than thionyl chloride for preparation of volatile acid chlorides like acetyl and propionyl chlorides.
Organikum specially mentions that thionyl chloride is not suitable for the preparation of those due to high losses with the SO2 and HCl offgas and
high residual SOCl2 content of the acid chlorides.
Benzoyl chloride can be made from benzotrichloride and benzoic acid with ZnCl2 as catalyst. I once opened a thread about this here and a patent for
this process had been posted there.
One can even make acetyl chloride directly from benzotrichloride and acetic acid with ZnCl2.
Benzotrichloride is made by chlorination of toluene at reflux and under strong light.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You can also chlorinate benzoic acid or sodium benzoate to the benzoyl chloride with TCT alone or TCT/DMF.
Not toat it does people much good where TCT is hard to obtain.
Except, see the thread on CS2 + Cl2 and find out how to make CC from Cl2 and MeSCN. Along with other useful products.
If you can buy or make a methyl halide and buy KSCN or NaSCN you can make MeSCN easily.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Acros sells benzoyl chloride and benzoic acid for roughly same price. And it's rather cheap.
I'll see what a 25 Kg sack of the technical benzoic acid goes for here. Then figure the cost of making a Kg of benzoyl chloride from that and TCT/DMF.
Then compare that to the landed cost of the Acros benzoyl chloride which from experience is about 2X the catalog price. That would be in vicinity of
$40-$45 US a Kg.
Then compare the cost of using TCT directly to make propionyl chloride to the cost of doing same with benzoyl chloride.
TCT does have 3 active chlorines, benzoyl chloride only one, and that will tend to make a difference in molar rations and thus cost.
I'm not a TCT salesman and have no particular ax to grind, or as say, no dog in this fight. I'm as interested in finding the best routes to acid
chlorides and anhydrides as anyone else.
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
What is the difference between Cyanuric chloride (CC) also called TCT or trichloro-s-triazine and trichloro-s-triazinetrione?
I have tens of kilo's of the latter............
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
"Trichloro-s-triazinetrione" is just a bad name for trichloroisocyanuric acid.
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
I see
1,3,5-Trichloro-2,4,6-trioxohexahydro-s-triazine; 1,3,5-Trichloro-s-triazine-2,4,6(1H,3H,5H)-trione; 1,3,5-Trichloro-s-triazinetrione;
1,3,5-Trichloroisocyanuric acid; Isocyanuric chloride; Symclosene; Trichlorocyanuric acid; Trichloroisocyanuric acid;
1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro-; s-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro-
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Two different but related compounds with very different properties.
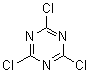
Cyanuric chloride is trichloro-s-triazine, the trimer of cyanogen chloride. The chlorines are attached to carbons.
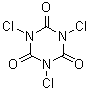
Trichloroisocyanuric acid, in contrast, has the chlorines on the nitrogens.
CC is used industrially to manufacture agricultural pesticides.
Trichloroisocyanuric acid is mainly used as a swimming pool chlorinator.
CC One of my favorite molecules because it is so useful. Acid chlorides from acids, acid anhydrides therefore by extension. Alkyl chlorides from
alcohols. In concert with KBr, alkyl bromides from alcohols.
To give credit where it is due I first learned of this one on Rhodium maybe 5 years ago.
The other I am becoming more fond of, I only encountered it two months ago here. Live and learn.
[Edited on 9-2-2007 by Sauron]
[Edited on 9-2-2007 by Sauron]
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
Thats what I thought,
so trichloro-s-triazine is not the same as trichloro-s-triazinetrione
or
Cyanuric chloride is not the same as Trichloroisocyanuric.
For a minute there I thought my pool chemicals had other uses ( which they do just not this )
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
[108-77-0]
C3Cl3N3
[Edited on 9-2-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
[87-90-1]
C3Cl3N3O3
[Edited on 9-2-2007 by Sauron]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I found benzoyl Cl to be cheaper than TCT. Thanks vey much. Looks like the SOCl2 method gives shitty yields. The acid is cheap the SOCl2 is not and
best be saved for higher weight acids.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
TCT is about $2 a Kg cheaper, but has 3 active chlorines to benzoyl chloride's 1, and does not have to be used in excess. Stoichiometric proportions
are fine. Normally you figure only two of the chlorines will be used, so to chlorinate a monobasic acid you'd use 1 mol TCT to 2 mols of acid. 1:2 not
2:1.
With benzoyl chloride you are advised per that JACS article to use a 50% to 100% excess (3:2 or 2:1) on molar basis of the benzoyl chloride vs the
acid substrate.
Half a mol TCT to do one mol of acid, versus 1.5 to 2 mols benzoyl chloride to do same.
That is 3-4 X more reagent to do same job on same acid and, does not bode well for the cost effectiveness of benzoyl chloride vs TCT.
Harder to quantify are matter of ease of workup, etc.
At that point it becomes a matter of personal preference.
Acros sells CC (TCT) for $30 a Kg, and benzoyl chloride for $27 a liter. d1.2 so that liter is 1200 g. Still...3-4 to one.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
TCT looks pretty attractive. What is the workup?
|
|
|
| Pages:
1
2
3 |