Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Making dichromate with sodium percarbonate
As I was making Fe2O3 from stainless steel, I wondered if it would be possible to turn the chromium in to dichromate - making
two useful things at once!
My process is electrolysis of NaCl with a stainless steel anode at 3V/2A.
The corrosion rate is rather high.
Then I filtered off the remaining "mud".
Until now, I would let this dry and then blast it with heat to make iron(III)-oxide (quite impure as it is contaminated with nickel and chromium).
But wouldn't it be possible to treat the "mud" with sodium percarbonate (https://en.m.wikipedia.org/wiki/Sodium_percarbonate) to turn any soluble iron ions into iron(II)-carbonate and the let the
H2O2 oxidize the Cr(OH)3 into soluble chromate? Acidifying the filtered solution makes sodium dichromate - but I
think the high solubility is a problem...
This source notes the use of 30% hydrogen peroxide as a way to go, but not with the percarbonate: http://amateurchemistry.weebly.com/making-potassium-dichroma...
My source of 2Na2CO3 • 3H2O2 is only 30% by weight. Not sure if it would work.
Opinions?
we apologize for the inconvenience
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I don't see why that wouldn't work. I imagine that the chromium would oxidize faster if you add sodium hydroxide to help leech it from the mud.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Seems plausible. The NaOH could promote the formation of soluble chromium [Cr(OH)6]3-
Found this interesting page about the chemistry of chromium: http://www.chemguide.co.uk/inorganic/transition/chromium.htm...
we apologize for the inconvenience
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
More precisely, you are not working with 2Na2CO3 • 3H2O2. See my commentary at http://www.sciencemadness.org/talk/viewthread.php?tid=73626#...
It could very well be, per my research, actually something much stronger and nasty, hence the necessity of the curious severe warning labels for what
you think is just H2O2 and washing soda.
The good news it may work better for you, but the brew with chromium salt could undergo a REDOX to Cr(Vl), so be wary. See my commentary at http://www.sciencemadness.org/talk/viewthread.php?tid=77197#... .
[Edited on 5-10-2017 by AJKOER]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That is a good page. Chromium is a fun element. Chromium compounds can take on any color of the rainbow, and they are useful too.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Thank you for the links.
Rather strangely I didn't notice any odd warning labels on my percarbonate. It just shows an exclamation mark and says basically "Keep out of reach of
children" and "Avoid eye contact", in the latter case saying you should immediately wash your eyes and go to a doctor.
In short it is almost identical to the warning label on my Na2CO3.
But the amount of TAED should be small, right? It isn't even listed, as you have written, and I can confirm that.
we apologize for the inconvenience
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
From what I've read, it seems that TAED is used in laundry detergents,however sodium percarbonate isn't meant to be used by itself as a detergent;
it's meant to be used as an additive, or for other cleaning purposes. If it's white and odorless, that would certainly be a good sign though.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Well, I consider that good news!
I compared two products containing sodium percarbonate, and the one I have good access to seems to be rather pure.
I didn't find a MSDS for the crappy one, but it has colored things in there, smells like washing powder and has some heavy duty warning stuff on the
box.
(See the attachment)
For the better one, it is pure white, has no colored stuff and smells like nothing. The warning labels are not as severe either.
It is even advertised as being "pure oxygen bleach".
Both say ">30% sodium percarbonate".
But according to the MSDS of the second one it has 40%-50%! Even better!
https://www.reinigungsberater.de/dokumente/sauerstoffbleiche...
And it is pretty cheap, ~ 8€ / kg.
Overall it seems like there are major differences between the brands in terms of purity.

[Edit reason: Replaced shortend link]
[Edited on 6-10-2017 by Diachrynic]
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I finally got a hotplate (without stirring though), so I'm looking forward to actually doing this experiment the following days. :D
One point that still bothers me:
Let's say I wash my mud to remove the salt, then I get a cup of the washed oxides/chlorides/hydroxides and add it to a heat resistant flask. Then I
would add my percarbonate and some more water to dissolve all.
It would fizz with carbon dioxide and oxygen as breakdown products of the carbonate and the peroxide respectively.
I would then boil it after a certain time to drive the reaction forward and break down remaining peroxide.
However, wouldn't the soluble Fe3+-ions have a happy time catalytically breaking down my peroxide before it has a chance to oxidize the
chromium?
[Edited on 12-11-2017 by Diachrynic]
we apologize for the inconvenience
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You don't want chlorides in the mix. Some oxides are probably unavoidable.
The iron salts shouldn't be very soluble, but it does seem likely that you'll need to add an excess of percarbonate.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Update: Good news!
Did a test tube scale test. Looking good. Very good.
About 10 ml of oxide sludge was added to a test tube. About 10 ml of distilled water were added. About half a gram, maybe a bit more of NaOH was
added. Not much heat evolved.
Next a water bath was set up, warm, dunno maybe 50-70 C, didn't bother to measure temp.
About a gram, maybe two, of percarb were added slowly. Gas produced, probably oxygen of decomposition of peroxide.
Kept in bath for about 5-10 minutes.
Greenish colour changed to brown, probably Fe(II) oxidized to Fe(III).
Filtered.
Filtrate is yellow coloured. Lovely cancer-causing colour of chromate.
Success! This is a proof of concept. 
Yield calculations and larger scale runs have yet to be done. Also, extraction/recryst.
But this is looking good! Salt, oxi-bleach and electricity can turn stainless steel into Cr(VI).
Will report again when more tests have been done.
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
There is some salt crystallizing out as it cooled to room temperature, can't possibly be chromate, because that is quite soluble and there is really
not much of it. Also, the crystals are clear/white-ish.
What could that be? My oxide sludge has been sitting in 2M NaOH for a month in PET, probably not one of my best ideas.
Could it be Na2CO3? Or something from the plastic?
Don't have any pictures for the moment.
we apologize for the inconvenience
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm not sure... it might be a calcium salt if you used tap water. I saw something like that when I made my calcium chromate.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Diachrynic  | There is some salt crystallizing out as it cooled to room temperature, can't possibly be chromate, because that is quite soluble and there is really
not much of it. Also, the crystals are clear/white-ish.
What could that be? My oxide sludge has been sitting in 2M NaOH for a month in PET, probably not one of my best ideas.
Could it be Na2CO3? Or something from the plastic?
Don't have any pictures for the moment. |
It's Na2CO3*10H2O most probably as that doesn't have great solubility at room temperature.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I've found a number of pages that explain a number of ways to get various dichromates from iron chromate (usually starting with stainless steel).
These pages might be worth a look to get some ideas of how it is done. A number of them use Na2CO3 to make sodium chromate/dichromate.
Some of the sites also have some good threads that are worth looking over as well, especially the first link.
https://parazite.nn.fi/roguesci/index.php/t-4144.html
http://amateurchemistry.weebly.com/making-potassium-dichroma...
https://www.askiitians.com/forums/Physical-Chemistry/describ...
https://byjus.com/chemistry/potassium-dichromate-and-potassi...
http://www.saralstudy.com/study-eschool-ncertsolution/chemis...
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by CobaltChloride  | Quote: Originally posted by Diachrynic  | There is some salt crystallizing out as it cooled to room temperature, can't possibly be chromate, because that is quite soluble and there is really
not much of it. Also, the crystals are clear/white-ish.
What could that be? My oxide sludge has been sitting in 2M NaOH for a month in PET, probably not one of my best ideas.
Could it be Na2CO3? Or something from the plastic?
Don't have any pictures for the moment. |
It's Na2CO3*10H2O most probably as that doesn't have great solubility at room temperature. |
Plausible, but I doubt it if there are other sodium salts in solution, if only because I did an experiment making sodium chromate once with an excess
of sodium carbonate in solution, and it precipitated very cleanly as a lower hydrate. Then again, I don't think decahydrate crystals form at all in a
warm room... if the crystals dissolve easily in water and react with acids to give off a gas, they are probably sodium carbonate.
[Edited on 24-3-2018 by JJay]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Thank you all for your replies and links! I figured it is probably some hydrate of sodium carbonate - makes the most sense to me.
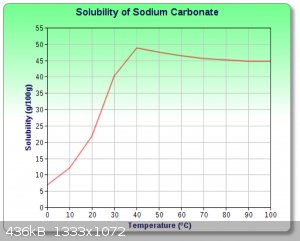
Will do some tests tomorrow.
[Edited on 24-3-2018 by Diachrynic]
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
My shed has room temp of 4 C, sadly.
we apologize for the inconvenience
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Should be easy enough to test.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
I recently made potassium dichromate from stainless steel you should see that video on my channel . I have used measured quantities of various
chemicals .
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Yeah, I've seen that video. It is really cool, but not OTC for me. Finding nitrate OTC is kind of hard (maybe I'm not looking in the right places) for
me.
The two most OTC sources of nitrates are a) firecrackers (which are a fucking horrible source I must say and a waste of time and money as well,
seriously, don't extract KNO3 from black powder, its like MnO2 from batteries...) and b) sparklers containing the barium salt.
I figured a complete easy (as easy as it gets obviously) OTC-method would be the best.
I see it as a kind of challenge. I don't think anybody has done this before. At least I read it nowhere. Just peroxide, no percarbonate. But the
latter is OTC in kilo quantities. And cheap. Indeed very cheap.
we apologize for the inconvenience
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
eBay.de has NPK fertilizer/rasendunger
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Diachrynic  | Yeah, I've seen that video. It is really cool, but not OTC for me. Finding nitrate OTC is kind of hard (maybe I'm not looking in the right places) for
me.
The two most OTC sources of nitrates are a) firecrackers (which are a fucking horrible source I must say and a waste of time and money as well,
seriously, don't extract KNO3 from black powder, its like MnO2 from batteries...) and b) sparklers containing the barium salt.
I figured a complete easy (as easy as it gets obviously) OTC-method would be the best.
I see it as a kind of challenge. I don't think anybody has done this before. At least I read it nowhere. Just peroxide, no percarbonate. But the
latter is OTC in kilo quantities. And cheap. Indeed very cheap. |
I wanted to tell you that barium nitrate from sparklers is a bad source. I scraped the powder of a sparkler and stirred it into hot water. After a bit
of stirring, I filtered the residue (mostly Fe powder). I added to the solution a solution of magnesium sulfate, but only a very small amount of
precipitate formed.
I got my nitrates as a 5 kg bag of pure NH4NO3 (for only 2.14 euro as well!) from a small fertilizer and pesticide shop located in the industrial area
of my town. You might want to check small shops around town and ask for "pure nitrogen fertilizer". I'm sure you'll find one that sells it as long as
you try to look like a gardener and not like a terrorist.
I think you should check out Extractions&Ire's video series on making dichromates/chromates (https://www.youtube.com/watch?v=F_W-IyUTM5M https://www.youtube.com/watch?v=3uzTNjuUyyk). It is pretty OTC. If you can't find HCl in shops (it is quite rare in Europe), you should go to
small car parts shops and ask for car battery electrolyte. This is H2SO4 and can replace HCl in Extractions&Ire's method.
[Edited on 25-3-2018 by CobaltChloride]
[Edited on 25-3-2018 by CobaltChloride]
[Edited on 25-3-2018 by CobaltChloride]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Quote: Originally posted by CobaltChloride  |
I wanted to tell you that barium nitrate from sparklers is a bad source. I scraped the powder of a sparkler and stirred it into hot water. After a bit
of stirring, I filtered the residue (mostly Fe powder). I added to the solution a solution of magnesium sulfate, but only a very small amount of
precipitate formed.
I got my nitrates as a 5 kg bag of pure NH4NO3 (for only 2.14 euro as well!) from a small fertilizer and pesticide shop located in the industrial area
of my town. You might want to check small shops around town and ask for "pure nitrogen fertilizer". I'm sure you'll find one that sells it as long as
you try to look like a gardener and not like a terrorist.
I think you should check out Extractions&Ire's video series on making dichromates/chromates (https://www.youtube.com/watch?v=F_W-IyUTM5M https://www.youtube.com/watch?v=3uzTNjuUyyk). It is pretty OTC. If you can't find HCl in shops (it is quite rare in Europe), you should go to
small car parts shops and ask for car battery electrolyte. This is H2SO4 and can replace HCl in Extractions&Ire's method.
|
About the nitrate: Damn, that is a good buy! I'll start looking, but I have not much hope. Nitrates are getting rare I feel. At least where I live.
About the barium: I don't know, technically they should be half nitrate by weight. Maybe they throw in other nitrates? I have a solution from half a
year ago that should contain about 4 grams of sparkler-nitrate. Haven't had any time to boil it down.
Thanks for the concern. It could very well be even that isn't an option for me then :/
About Ex&I: Yeah, I've seen that video as well! Really good. Unfortunately, I'm currently searching for bleach as well.
In conclusion I'm running quite low on... well, everything, really. Even time.
we apologize for the inconvenience
|
|
|