| Pages:
1
2
3
4
5 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I'd mostly like routes that start from things that I have, and I currently have about a pound of ethylvanillin that I have absolutely no use for.
Also, 100 grams of regular vanillin and 100 grams or so of catechol. The Duff reaction does seem like it's low-yielding even when it's used for the
reactions it works well with. Formylating catechols doesn't seem to be a reaction it's used for though, and in a paper I read, the authors actually
claimed that it wasn't worth doing the workup to figure out what the yield was.
Am I supposed to use anhydrous HBr for dealkylation? I actually found a source of pyridine that isn't outrageous:
https://www.chemsavers.com/p/pyridine-99-2-5l/
2.5 liters for $100. Odd that I haven't thought to look there recently for anything. They're a little on the expensive side for reagents in general,
but they have a huge array of chemicals that I doubt I'd find anywhere else. Incidentally, they're the only supplier that sells to the public that I
know of, where it's possible to get TMSCI, although they have it listed as "chlorotrimethylsilane":
https://www.chemsavers.com/search.php?search_query=%22chloro...
However, other tertiary amines besides pyridine are also supposed to work, so I'll probably try TEA before I go ordering anything.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Melgar  |
I have a lot of aluminum that's alloyed with gallium and other metals, and is attacked very easily. I've used it to make aluminum triiodide by adding
iodine to a nonpolar solution with this aluminum alloy in it. Is that something I could do with HCl gas, to get anhydrous AlCl3? Only one way to
find out, I guess.
[some time later]
Okay, well the only solvent that seemed to work was diethyl ether. Bubbled HCl into that, with a chunk of activated aluminum, and it bubbled quite a
lot, then formed a film on the glass, and the index of refraction of the solution went up noticeably. Interesting...
[Edited on 10/29/17 by Melgar] |
I've seen a lot of AlCl3 manufacturing attempts in other solvents on here, but I haven't found any yet that have worked well. This might be fairly
important in and of itself. At least worth posting as its own thread (or on some AlCl3 thread)so it's easy to find.
Would this stuff be reasonably safe to evaporate if the ether used was peroxide free?
I did a little poking around and US3032508A says even the ether/AlCl3 complex itself works in FC reactions if the AlCl3 content is high enough
(between 1 and 2 moles AlCl3 per mole of ether).
Maybe the complex would function as AlCl3 in some other reactions too.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by SWIM  | I've seen a lot of AlCl3 manufacturing attempts in other solvents on here, but I haven't found any yet that have worked well. This might be fairly
important in and of itself. At least worth posting as its own thread (or on some AlCl3 thread)so it's easy to find.
Would this stuff be reasonably safe to evaporate if the ether used was peroxide free?
I did a little poking around and US3032508A says even the ether/AlCl3 complex itself works in FC reactions if the AlCl3 content is high enough
(between 1 and 2 moles AlCl3 per mole of ether).
Maybe the complex would function as AlCl3 in some other reactions too. |
The hard part was finding a solvent that wouldn't react with aluminum metal, but would dissolve significant amounts of AlCl3. It can't be aromatic,
of course, because then the FC reaction starts in. Acetone and MEK both reacted with the aluminum, which surprised me a bit. Of course, there would
probably be a bunch of HCl dissolved in the ether, and I think that might be a good thing for FC reactions, no? It'd probably help this particular
dealkylation procedure, even.
HCl was generated via H2SO4 and MgCl2, so it should be relatively anhydrous. I stop the reaction by adding water, after all.
I'd post something when I have more to post, I suppose. The aluminum was by no means pure, but all the other metals should either be inert or assist
with the reaction, I believe.
Doesn't the FC reaction normally occur in ether? That's one of the reasons I thought to use it. There really aren't that many solvents that are
inert enough for the reaction's conditions and also will dissolve AlCl3.
[Edited on 10/30/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I've mostly seen FCs done with an excess of the substance to be alkylated as the solvent to suppress over alkylation, but I haven't seen that many, so
my experience isn't a good indicator. And they probably tend to shy away from reactions nowadays that call for huge excesses of benzene. I've only
done one with ferric chloride (or zinc? I think it was iron, but would rather not quote that particular journal article here...)
The patent I saw was using the complex made from ether and AlCl3 rather than generating it in situ, so I wasn't thinking about the excess HCl in there
in your case, But your thoughts on that sound good to me(for what that's worth).
I'm not trying to talk you into trying anything unconventional for your current project; as you said, best to go for stuff you can rely on as known
quantities.
Just wanted to point out you might have found something very useful that might not be covered on SM.
The comment about the ether/AlCl3 complex was just a side thought really. Probably easier to evaporate the solution to dryness rather than titrating
it for use as is. I just have a bit of a phobia about boiling anything in ether to dryness . .
|
|
|
chemplayer...
Hazard to Others
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Forgot to mention, this was another interesting potential reaction which could give the dopamine / l-dopa side-chain:
https://www.youtube.com/watch?v=ExVoMqHDFxM
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
I gave it a thought once... came up with this:
Step 1:
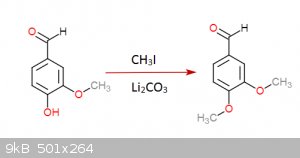
Step 2:
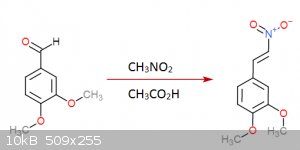
Step 3:

Step 4:
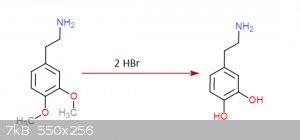
Each step has its reference... I was an idiot and didn't save them but I could look them up (Not on these molecules of course but in rather similar
ones)... the funny thing about this is that step 3 is a rather easy hydrogenation that can be done at practically normal room conditions, the bad side
though is that palladium acetate is quite expensive... you don't need much though and is not very hard to get either.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In this paper, vanillin is reductively aminated with a secondary amine and the product reacts with potassium cyanide in DMF at 120 C to give a
phenylacetonitrile via an intermediate quinone methide. The phenylacetonitrile is reduced to the phenethylamine and the product 3O-methyldopamine can
be demethylated easily.
http://www.sciencedirect.com/science/article/pii/00404020738...
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Allylation of catechol is applicable to dopamine, by the next scheme:
mono-O-Allyl-catechol => 4-Allyl-catechol => (double bond cleavage) => Ar-CH2-CHO => (reductive amination) => Ar-CH2CH2NH2 (=dopamine)
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by Outer  |
Allylation of catechol is applicable to dopamine, by the next scheme:
mono-O-Allyl-catechol => 4-Allyl-catechol => (double bond cleavage) => Ar-CH2-CHO => (reductive amination) => Ar-CH2CH2NH2 (=dopamine)
|
Serious? And where did the other carbon goes? Desappeared by magic?
Do you mean Ozonolysis of the allyl group? It's the only way I know to do this kind of cleavage, but it's not OTC at all.
EDIT: Interesting. I found a recipe to cleavage the double bond with KMNO4 in THF from Rhodium pages. May be it's possible do the cleavage of allyl
cathecol in 3,4 Hydroxy Phenyl acetaldehyde and formaldehyde like you said:
https://erowid.org/archive/rhodium/chemistry/alkene2aldehyde...
[Edited on 7-11-2017 by Chemi Pharma]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
@BlackDragon2712 For the demethylation, is there some trick or procedure, assuming you have 48% hydrobromic acid? I'm having trouble finding a good
descriptive procedure.
@not_atara Wow. That is a very interesting combination of reactions. The alkali cyanide isn't ideal, but I recently read a 19th century chemical
engineering text that described "fusing" a mixture of (I think) sodium sulfide with postassium ferricyanide or potassium ferrocyanide, and getting a
molten mixture of NaCN and KCN with the FeS precipitating from the melt. I have plenty of KCN, so it wasn't that useful for me, but I'll have to see
if I can find that again. The text claimed that this ratio had a lower melting point, and that for applications where either alkali cyanide could be
used, that this mixture was especially convenient.
@Outer I feel like I should reply that the Mannich reaction would be a better way to go than allylation and subsequent ozonolysis or whatever, but I
guess I'm not familiar enough with the Mannich reaction to say that with 100% certainty.
I'm toying with the idea of using this reaction to illustrate the vast array of options available for certain syntheses, and encourage the sort of
lateral thinking that makes for good organic chemists. Also, emphasize the vastness and variation of organic chemistry; it's not "chemistry of only
one element" any more than computer programming is "math with only two numbers". This thread has certainly given me plenty of material. After all,
if the goal is education, emphasizing the large variety of potentially useful reactions is probably the most educational way of framing it. It'd have
to be split up into a bunch of videos, but that's not a problem.
[Edited on 11/7/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by Melgar  |
@not_atara Wow. That is a very interesting combination of reactions. The alkali cyanide isn't ideal, but I recently read a 19th century chemical
engineering text that described "fusing" a mixture of (I think) sodium sulfide with postassium ferricyanide or potassium ferrocyanide, and getting a
molten mixture of NaCN and KCN with the FeS precipitating from the melt. I have plenty of KCN, so it wasn't that useful for me, but I'll have to see
if I can find that again. The text claimed that this ratio had a lower melting point, and that for applications where either alkali cyanide could be
used, that this mixture was especially convenient. |
Here's the link to get the paper Atara said in PDF:
Synthesis of phenethylamines from phenylacetonitriles obtained by alkylation of cyanide ion with mannich bases from phenols and other benzylamines.
J.H.Short, D.A.Dunnigan, C.W.Ours
http://chemistry.mdma.ch/hiveboard/rhodium/pdf/pea.mannich.b...
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
@ChemiPharma Yep, I'd gotten it already from sigh'ubb, but good to have the link. Also, the other way to cleave double bonds that way is by
converting to the diol, then reacting with periodate. Not saying it's especially practical, but it might be of some interest down the road.
IIRC you can make sodium periodate just by adding enormous amounts of bleach to elemental iodine. Or, if you add iodine to NaOH for the Finkelstein
reaction, you could probably just separate out the NaIO3 and oxidize that with bleach. I think it can be made by electrolysis of an alkali iodide
salt, but I'm unclear on the details.
Also... "recipe"? You should really know better by now. 
[Edited on 11/7/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Melgar  | @BlackDragon2712 For the demethylation, is there some trick or procedure, assuming you have 48% hydrobromic acid? I'm having trouble finding a good
descriptive procedure.
|
Well... I'm going to be plain honest here, I lost all references but for step 2 and 3 a long time ago  ... though it is by no means conclusive if it works in this case, the cleavage of methoxy group in guaiacol with HBr
is described in Org Syn. here: ... though it is by no means conclusive if it works in this case, the cleavage of methoxy group in guaiacol with HBr
is described in Org Syn. here:
http://www.orgsyn.org/demo.aspx?prep=CV1P0149
... The double cleavage of a cathecol like ring is described in page 7 of this link:
https://www.thevespiary.org/library/Files_Uploaded_by_Users/...
I would expect HI to be better in cleaving alkoxy groups...
On a side poorly relevant note though, on this book it's mentioned that LiCl apparently can cleave aromatic methoxy groups on refluxing in DMF for
4-72 hours... though vaguely described it does seems interesting...
https://books.google.cl/books?id=B2BEBAAAQBAJ&pg=PA550&a...
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Safer hydrogenation protocol:
http://onlinelibrary.wiley.com/doi/10.1002/chem.201001377/ab...
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Chem Player did this video on cleaving the double bond in piperinic acid using a combination of permanganate and periodate: https://www.youtube.com/watch?v=6r9elLR2WjI
The workup would be more somewhat complicated for 4-allyl catechol but is probably not outrageously difficult. If I'm understanding the
periodate/glycol oxidation mechanism, it would occur on the order of a million times slower on the catechol phenol groups than on a phenyl 1,2-diol.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I forgot if this has been posted or not, but this paper describes reduction of nitrostyrenes to dopamine analogs with just zinc and hydrochloric acid
in methanol:
https://www.researchgate.net/publication/274084261_Chemosele...
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Oh god, this may make the vanillin pathway quite easy if it works!! great finding!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemi Pharma  |
Do you mean Ozonolysis of the allyl group? It's the only way I know to do this kind of cleavage, but it's not OTC at all.
EDIT: Interesting. I found a recipe to cleavage the double bond with KMNO4 in THF from Rhodium pages. May be it's possible do the cleavage of allyl
cathecol in 3,4 Hydroxy Phenyl acetaldehyde and formaldehyde like you said: |
Quote: Originally posted by Melgar  |
@Outer I feel like I should reply that the Mannich reaction would be a better way to go than allylation and subsequent ozonolysis or whatever
|
Quote: Originally posted by Melgar  | | the other way to cleave double bonds that way is by converting to the diol, then reacting with periodate. |
Quote: Originally posted by JJay  | | Chem Player did this video on cleaving the double bond in piperinic acid using a combination of permanganate and periodate.The workup would be more
somewhat complicated for 4-allyl catechol but is probably not outrageously difficult. If I'm understanding the periodate/glycol oxidation mechanism,
it would occur on the order of a million times slower on the catechol phenol groups than on a phenyl 1,2-diol. |
Won't the catechol convert immediately to quinone in the presence of oxidizing agents ?
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm actually not 100% sure about that part, but I think that reactions that would produce hydroxybenzoquinone are several times slower than the
reactions that cleave the double bond and would take several minutes to hours to complete in the cold. That would likely be a competing reaction if
the hydroxyls aren't protected, though, and it's fast enough to significantly hurt yields.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Yes, there would be a problem related to oxidation of catechol fragment. But on the other hand, if it is oxidized to o-quinone, it will protect the
catechol from side reaction with formaldehyde (which is released during double-bond cleavage). After removal of formaldehyde, o-quinone fragment can
be reduced back to catechol.
[Edited on 8-11-2017 by Outer]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I actually feel pretty silly, because I thought I'd posted it a few pages back, but apparently didn't. The nitrostyrene reduction procedure was
written up superbly, although it annoyed me a bit right at the end where they pull out BBr3 to dealkylate to dopamine. I'm left wondering what these
side-reactions were that their lengthy workup avoided? My guess would be having the ZnCl2 acting as a FC catalyst or similar, considering they were
using halogenated substrates, in which case it might not be necessary to be QUITE as careful as they were.
I guess that'll have to be determined via trial and error though.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Boron tribromide doesn't look that outrageously difficult to make. I'm not seeing a convenient lab-scale synthesis written up anywhere, but it does
not look at all out of reach of the home chemist.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Regarding the Mannich base/cyanide route, I'm pretty sure that a Mannich rxn on guaiacol yields predominantly isovanillylamine, so to get
vanillylamine you need to do a reductive amination on vanillin or use some kind of catalyst. However, I'm not sure. This paper probably has details:
http://pubs.acs.org/doi/abs/10.1021/ja01145a017?journalCode=...
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Outer  | | Yes, there would be a problem related to oxidation of catechol fragment. But on the other hand, if it is oxidized to o-quinone, it will protect the
catechol from side reaction with formaldehyde (which is released during double-bond cleavage). After removal of formaldehyde, o-quinone fragment can
be reduced back to catechol. |
But it won't stay o-quinone,would it ? It would furthur polymerise to form tarry crap
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
If you can add and remove protecting groups without a lot of time or expense in high yields... sure.
But not everyone wants to drip bromine through a 325 C tube loosely packed with boron carbide and deplete their stocks of methyl iodide just to
improve yields. It depends on how big the yield improvement is.
|
|
|
| Pages:
1
2
3
4
5 |