| Pages:
1
2
3
4 |
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I hope to get another gas cylinder today or tomorrow, I hadnt spotted a small leak! Which explains how I got through so much gas so quickly!
I wont be making drops for at least 4-5 days though, my lab needs sorting and i got other stuff i want to finish!! Plus after tomorrow some pics
showing my new upgraded indoor lab! No fume hood in that one, and it isnt any warmer though lol. But its mainly for the biochem stuff. I wanted to
separate the stuff I am doing.
But once i get that out the way, i am back on the drops!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Today i tried both borosilicate sirring rods, a smashed up jam jar and a window pane.
The jam jar glass got all bubbly rather than melting into a transparent blob.
The window pane just kept cracking and falling to bits before melting.
Stir-rods melted much more easily and were far easier to handle.
Making the tails seems easy enough, just the heads all broke up on cooling.

If anyone gets good at making the heads without a tail, i got spares 
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I've only attempted 4 drops but one was kind of lumpy when it separated from the tubing. As it fell into the water and cracked it seemed there wasn't
enough homogeneity to the shape and uneven stresses may have caused it to fracture. Rotating the tubing as the blob formed with not enough heat to
really melt the glass thoroughly, it just kept sagging and it may be that a hotter flame would be easier to get a uniform melt and thus less cracking
on cooling.
I started out with a basic propane torch with a pencil flame and then used another torch with a slightly more bushy flame, neither of which lent
itself to doing any borosilicate melting in a timely manner, even using two at the same time, finally settling on MAPP gas and that piezo spark torch
head with the noisy flame. If I had a choice, I'd opt for a propane/oxygen flame or acetylene.
Trying to melt some obsidian collected from Oregon, I noticed it's really hard to melt and it shatters like ordinary glass unless I guess you slowly
walk the temperature up.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | Painted the inside of cupboards as a joke? Not a joke your victim would survive if that were literally true, and the idea of a paint can full is
insane.
A couple of grams was enough to ruin the stuff on my desk top as a child.
It CAN go off while wet. The nice purple smoke hanging after it goes off is elemental Iodine, not something good to breathe
This material is decidedly in the category of "everyone who tries it has an accident".
It goes off from the vibration of closing a door to the room it was drying in. You can't safely move it when it is dry.
[Edited on 31-12-2017 by Bert] |
He was on most episodes, his name is peter Logan.
https://www.youtube.com/watch?v=F0-jq2eJBYA
Watch from 19:34 for a min or 2, that episode is pretty mild. In some of them you see the can nearly full and he puts loads on under carpets etc. I
remember the program from way back! It was a kind of science program for kids!!
Except once you start learning science you realize the program is shit!! They even tell you its made from Iodine and " one other secret chemical".
didnt take long for people to figure out what that was!
I guess his victims got lucky, you can see from the reactions they didnt know what was coming.
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Saw this one tonight with very long tails.
https://www.youtube.com/watch?v=v8OK2FC440w
And this one with a Leidenfrost Rupert drop in slow motion nicely done.
https://www.youtube.com/watch?v=1z5rUvvRJuI#t=3m57s
[Edited on 3-1-2018 by Morgan]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If MAPP gas is marginal for working larger rods of glass, I am going to be needing a bigger torch. All I have now is a basic plumbers type propane
torch, no Oxygen. Took a look at gas welding equipment, pricy... (we are all electric, have not touched oxy acetylene cutting or welding for years,
since I cleverly managed to break both my ear drums and ended up in a river).
Years ago, I used propane with compressed air fed through a standard oxy acetylene torch body and a variety of large, flame spreading tips at the
university physical science labs when working glass. It was adequate for quite large pieces and cheap.
[Edited on 5-1-2018 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice idea.
My tiny bottle of oxygen ran out earlier, yet i do have a compressor.
Will give it a try with butane.
Pray tell how the river, eardrums and oxy/acetylene became entwined.
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
If you put a glass rod up the tailpipe of a pulse jet, you could probably melt it that way. And by some means if the ejected blob-like mass was
captured in a nearby bucket of water below the exhaust, maybe there'd be a chance to form some drops akin to how volcanoes sometime eject glassy
fragments into the air that cool into such shapes. Well, it looks good on paper. ha
https://www.youtube.com/watch?v=F8vqfphZ73A
https://books.google.com/books?id=Pt5QAAAAYAAJ&pg=PA42&a...
[Edited on 5-1-2018 by Morgan]
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | If MAPP gas is marginal for working larger rods of glass, I am going to be needing a bigger torch. All I have now is a basic plumbers type propane
torch, no Oxygen. Took a look at gas welding equipment, pricy... (we are all electric, have not touched oxy acetylene cutting or welding for years,
since I cleverly managed to break both my ear drums and ended up in a river).
Years ago, I used propane with compressed air fed through a standard oxy acetylene torch body and a variety of large, flame spreading tips at the
university physical science labs when working glass. It was adequate for quite large pieces and cheap.
[Edited on 5-1-2018 by Bert] |
Bert, a swirl torch with MAPP should do fine. The swirl torch flame hugs the rod even on the backside. They are 30-50 dollars at home depo, the
swirl design really does work quite better than a standard design, it is somewhat new, last 5 years or so. If this is still not hot enough, which it
should be, then placing some ceramic insulation around the part you are heating will make an amazing difference.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Just made a few of these Ruperts drops from jam jar glass shards (prob. soda lime) and a propane burner. Very thin shards seem to work best, as larger
shards form larger droplets and droplets larger than 6-7 mm seem to disintegrate gradually during the cooling in water, maybe exceeding its own
tensile strength upon contraction. Got a few for which the tail broke off easily as well (without exploding the droplet). This seems to happen when
the tail isn't cooled fast enough,which can easily happen, since it looses heat to the surroundings much faster than the larger droplet. When the tail
of the droplet is heated a second time and allowed to cool slowly in the air, it can be broken off without the droplet exploding. Heating the entire
droplet by pointing the flame upward slightly to heat the entire droplet (also the top) and keeping it just cm's from the cooling water to fall into
works all the time. Was curious if they would behave similar when broken underwater, but they seem to do so, so any influence of resonance in the
crack propagation as I mentioned earlier seems unlikely.
[Edited on 8-1-2018 by nitro-genes]
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Decided to have a go at this and got a couple of good ones but only from borosilicate glass. The sofa line I tried all disintegrated. I worked out
that water needs to be cool but not cold. A couple of the ones I made appear to have a bubble in the end.
Anyway now we have the goods how do we go about getting this to work? Further up I read that maybe a tiny amount of primary could be used to bust the
tail, setting off the drop which is placed in a small amount of confined ETN or other sensitive explosive. Is there an easy way to determine where is
best to break the tail to give out the maximum energy to the explosive?
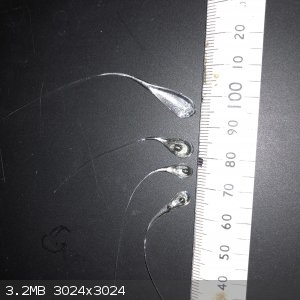
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Very nice indeed !
(jealous)
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NeonPulse  | Decided to have a go at this and got a couple of good ones but only from borosilicate glass. The sofa line I tried all disintegrated. I worked out
that water needs to be cool but not cold. A couple of the ones I made appear to have a bubble in the end.
Anyway now we have the goods how do we go about getting this to work? Further up I read that maybe a tiny amount of primary could be used to bust the
tail, setting off the drop which is placed in a small amount of confined ETN or other sensitive explosive. Is there an easy way to determine where is
best to break the tail to give out the maximum energy to the explosive? |
That one drop with the bubble right in the center reminded me of the evil eye, something I first saw and had as a young boy living on air force bases
in Istanbul and Peshawar, and recently when I took a glassblowing class the teacher was making them. But just now I came across this attribute or
supposed connection between evil eyes and Prince Rupert Drops. I'm sure most evil eyes aren't spring loaded of course, but it might be fun to try to
make one that way. Funny to be led to this article by your "evil eye" drop. I had heard of the protective belief but not of the exploding property.
You see I wondered if anyone had tried to make an evil eye Rupert drop as an artistic venture by coloring the glass and somehow strategic placement of
a bubble - thus a search "evil eye rupert drop" in Google this article came up as the first hit.
"Local legend has it that if an evil person enters your shop or home, the evil eye will spontaneously shatter, warning the owner to the presence of an
extremely evil person."
http://ieeexplore.ieee.org/document/1337917/
https://en.wikipedia.org/wiki/Evil_eye
[Edited on 9-1-2018 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
A few tidbits maybe of interest ...
How To Break a Wine Glass With Prince Rupert's Drop?
https://www.youtube.com/watch?v=akO6x5CF3f0
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
8:21 shows exactly what your looking for. Notice how all the best ones break into a powder?
Good video, it looks like the way forward, is find out what method makes drops that powder when they go off.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NeonPulse  | Decided to have a go at this and got a couple of good ones but only from borosilicate glass. The sofa line I tried all disintegrated. I worked out
that water needs to be cool but not cold. A couple of the ones I made appear to have a bubble in the end.
Anyway now we have the goods how do we go about getting this to work? Further up I read that maybe a tiny amount of primary could be used to bust the
tail, setting off the drop which is placed in a small amount of confined ETN or other sensitive explosive. Is there an easy way to determine where is
best to break the tail to give out the maximum energy to the explosive? |
If you look at the drop, you see where the rainbow colour ends as the drop start to form the tale. Directly behind that point is where they give the
best break.
If you break them too far from the rainbow colour (stress point), then they dont break well, you get more of a shatter and larger particles, or it
dosnt break the drop at all.
Make sure you got good eye protection! Actually working in water is not a bad idea.
[Edited on 15-1-2018 by NEMO-Chemistry]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Now the question is what can it set off from the glass explosion does it create a shockwave
Could it set off ammonium nitrate with sensitizer
Possible ones that are sensitive enough
With even Armstrong. Mix
Methylammoniunm nitrate
Or rdx which is a secondary
By going of how fast the drop explodes
[Edited on 15-1-2018 by symboom]
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I wonder if you mixed in some higher melting point chopped fused quartz wool into ordinary soda lime glass and then made drops from that, how would
the fibers affect the drop? Seems like it might dampen the effect if you could make them from that but who knows.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
MAPP torch should arrive tomorrow, I need to get my hands dirty.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by symboom  | Now the question is what can it set off from the glass explosion does it create a shockwave
Could it set off ammonium nitrate with sensitizer
Possible ones that are sensitive enough
With even Armstrong. Mix
Methylammoniunm nitrate
Or rdx which is a secondary
By going of how fast the drop explodes
[Edited on 15-1-2018 by symboom] |
If you study that last video really carefully, you notice it has one hell of a shockwave, its the shockwave you see travel up the drop, then as soon
as it touches the glass the glass breaks.
At first i thought it was more to do with the glass being at the right frequency, but the fact the glass has water in as well, that should have
dampened or altered the resonate frequencies, shouldnt it?
So if thats the case then the high speed film shows an enormous amount of power for a split second. I noticed with the few drops I have made, those
that glow in the water longest give the best results.
When they break, you can tell when you got it right by the powder formed. The finer the powder the better the drop. Those are the ones that sting lol.
The way the glass the bead is in breaks, I would think these would be more than capable of setting something off. I have a piezo electric strip,
setting it up so the bead touches it, the attaching it to an Oscilloscope, should gigve an idea how much force is there.
I suspect one of those piezo electric wafers, used in electronic drum kits, would also give a good idea of the energy produced.
I think for me thats the path I will go down, i can experiment with that, I dont think it makes any sense for me personally to use anything that goes
bang.
I know your looking to set off primaries, but with the test above, i should be able to compare what makes the best drop. So maybe next job is find a
way to make a more standard sized drop.
Also the break point on the tail seems important, but that could be coincidence. All these high speed films are really goo information.
I would love the full files for these, and go through them frame by frame, the problem is by the time they are converted to youtube etc, i bet they
have lost alot of information.
[Edited on 17-1-2018 by NEMO-Chemistry]
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Not all that exciting but it seems possible to "snap" a super saturated solution of sodium acetate into crystallizing using a Prince Rupert Drop
instead of oil canning a metal disk in a heat pack.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Well primaries already are sensitive I was thinking if there was enough energy to even replace a primary to set of a less sensitive secondary compound
it would be surprising if its possible.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
I was watching a few youtube vids with P Ruperts drops being fired at by small arms, in slow mo of course. What stands out is the deflagration of the
glass as being reasonably quick but not particularly energetic. The fragments don't seem to accelerate from the body of the fracture zone too fast,
compared to the fragments of the projectile. I guess you are looking at the stored energy as an elastic potential in all but a small skin layer of the
glass. Compared to the chemical energy stored in every molecule of a primary.
The energy density would be particularly low by comparison. I think there could be some novelty combination of substances that could form a
detonation train to initiate a secondary via a P Ruperts, but then a Rube Golberg machine could too.
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Maybe I missed the discussion somewhere but the fracture speed "1600 meters per second… or 3579.1mph" is always highlighted along with the tons of
force they can withstand but what speed do the glass fragments travel at? Seems like it should be mentioned next to the fracture speed to enhance the
understanding.
"So the end result is an awesome explosion of glass shards in all directions."
https://fabstracts.wordpress.com/2013/10/22/dutch-tears/
There's this sandbox pod that's said to explode with a speed of 66 meters per second and an angular velocity of 15,000 rpm in once case. It can break
windows and injure cattle when the pumpkin-shaped pod goes off with a bang. People in a forest have mistaken the explosions for gunfire.
It would be interesting to know how the angular speed of this seed compares to a Rupert Drop fragmenting? Or could you combine/idealize the angular
speed with the forward speed akin to being on the worst side of a hurricane when it hits - a contribution of angular motion and forward speed.
Sandbox tree disperses its seeds via explosive method
https://www.youtube.com/watch?v=AKEgrmfHWX0
http://onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.1977....
http://onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.1977....
[Edited on 19-1-2018 by Morgan]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Heuston, we have droppings !
2 cheapo butane/propane torches, a 250ml beaker of distilled water and a boro stirring rod worked first time, then three more times without fail.



It took a lot longer than the oxy/butane torch, so maybe that made them too hot.
Now to see if they go *pop* when the tail gets snapped off.
Edit:
Bugger. Not one of them went *pop*.
Must need to be a lot hotter before dropping into the water.
[Edited on 22-1-2018 by aga]
|
|
|
| Pages:
1
2
3
4 |