smartgene1
Harmless

Posts: 42
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
Can someone please help with chloramine hcl
Can someone please help with chloramine hydrochloride I know how to synthesis chloramine but I don't know how to make it a salt can some please help
I would appreciate it
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
How did you prepare the chloramine in the first place, ammonia and sodium hypochlorite?
If this is the case then you will likely have dichlorinated impurities in your gas flow.
To prepare the acid salt i would have assumed it would act like most other amines and simply react with HCl forming the salt.
Have you tried this?
Alternatively the chloride may steal enough of the electrons off the amine for the amine to be electronegative enough to form a sodium salt.
Im skeptical of this thought however as sodium hypochlorite is normally stabilized with an excess of NaOH and this would surely react with the
chloramine enough to prevent it from leaving the solution.
[Edited on 1-1-2018 by Assured Fish]
|
|
|
smartgene1
Harmless

Posts: 42
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
I made the chloramine with chlorine water and ammonia. I thinking of reacting chlorine with ammonium chloride to see what it would do
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Does chloramine form a hydrochloride?
Is it that good a bases?
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
That is a very bad idea. Chloramines will disproportionate into nitrogen trichloride at low pH. As can be found in a 10 second Google search
http://homepages.uc.edu/~maynarjb/Frontpage%20sites/603/Geoc...
https://www.lenntech.com/processes/disinfection/chemical/dis...
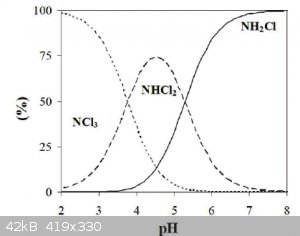
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
My source of assumption for chloramine hydrochlorides existence.
https://pubchem.ncbi.nlm.nih.gov/substance/341564608#section...
|
|
|