elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Synthetic Quartz Crystals - Alternate methods?
I recently became interested in producing synthetic gemstones from raw feedstocks. One of the ones I would like to eventually try is quartz,
specifically the colored varieties such as amethyst or possibly citrine.
The typical process for producing artificial quartz crystals is hydrothermal growth, which requires a carefully controlled temperature of something
like 300 to 400 degrees Celsius and a pressure of 700 to 1500 bars, likely to mimic the conditions required to form natural quartz crystals. However,
I can't for the life of me find a decent pressure reactor rated for such a high pressure, probably because 1500 bars is above the yield strength for a
decent portion of steel alloys (and well above the limit provided by Barlow's Formula for the reactor size I'd like to go for).
That in mind, does anyone know of any alternative method of growing quartz crystals, preferably at a much lower pressure? Or, better yet, a decent
source for cheap pressure vessels ('cheap' being within $1000, say)? I tried Alibaba, but that didn't yield much.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
If its water in your reactor at 400C why such high pressure?
It should only need no more than about 140bar.
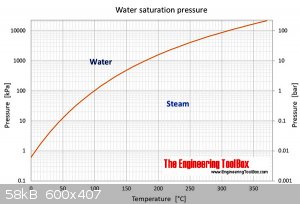
[Edited on 2-1-2018 by wg48]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by wg48  | If its water in your reactor at 400C why such high pressure?
It should only need no more than about 140bar.
[Edited on 2-1-2018 by wg48] |
Strange. Most of the literature I've read on hydrothermal crystal growth specifies an average pressure of 22,000 psi (1500 bar).
I think it might be to better mimic the natural conditions under which these crystals grow, though I'm beginning to wonder if that's truly necessary.
It's either that, or the growth rate increases at this increasing pressure.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | Quote: Originally posted by wg48  | If its water in your reactor at 400C why such high pressure?
It should only need no more than about 140bar.
[Edited on 2-1-2018 by wg48] |
Strange. Most of the literature I've read on hydrothermal crystal growth specifies an average pressure of 22,000 psi (1500 bar).
I think it might be to better mimic the natural conditions under which these crystals grow, though I'm beginning to wonder if that's truly necessary.
It's either that, or the growth rate increases at this increasing pressure. |
Apparently the pressure does change the solubility significantly particularly at super critical temperatures. As shown in the following graph from a
note by A. C. Walker.
But it will still work at lower pressures at lower sub critical temperatures just not as fast and apparently using carbonate.
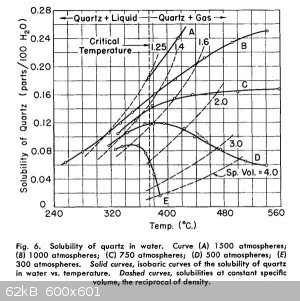
From:
Attachment: williams1940.pdf (206kB)
This file has been downloaded 1435 times
Attachment: walker1953.pdf (1.3MB)
This file has been downloaded 942 times
Sorry for the error the first file is about ketene prep but I have left it there as its interesting.
[Edited on 3-1-2018 by wg48]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Thanks! The graph and second paper were really helpful.
The second attached paper hints that the high pressures (1000 atm, in their case) are generated simply by heating the water inside the closed vessel,
without any special pressurizing apparatus. This probably means that if I hope to grow crystals at a reasonable rate, an autoclave capable of
withstanding that pressure is the only thing I really need to work towards.
Back to shopping around, I suppose...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Having thought about it, ethylene glycol dissolves quartz particularly with catalytic amounts of triethanolamine under relatively mild conditions.
Perhaps such a solution could be used to grow quartz crystals in a temperature gradient reactor.
See:Attachment: qudescheng2000.pdf (275kB)
This file has been downloaded 516 times
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That looks like it doesn't dissolve silica so much as react with it to form a soluble species- I have no idea if you'd be able to get the silica back
from solution.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | That looks like it doesn't dissolve silica so much as react with it to form a soluble species- I have no idea if you'd be able to get the silica back
from solution. |
As silica is a covalently bonded array of oxygen and silicon to get it to dissolve at near ambient condition it must be done by a reactive
solubilization just as the hydro thermal process does.
Presumably to get the silica back with a change in temperature the solubilization process must be reversible such that a temperature dependent
equilibrium exists. I don’t know if that’s true either.
However apparently from the note the reaction produces water and is driven by the water being removed by evaporation as the reaction conditions are
above the boiling point of water. That suggests there is equilibrium and if the equilibrium is temperature dependent then it may work.
Perhaps a sealed reactor using a glycol water mixture would work.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It might be interesting to try adding TEA to water + seeing if it improves the solubility at a more accessible temperature (It will also drop the
vapour pressure a bit).
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Having read that paper, I can't find any references to silica precipitating back out when the reaction mix was cooled. However, ethylene glycol and
silica are two easy-to-find things, so I can try this experiment out rather easily. Unfortunately, I lack any source of TEA - does anyone know of an
OTC product that contains it?
Alternatively, since it seems that ethylene glycol requires a basic pH to form the species responsible for dissolving quartz, could acidifying the
solution precipitate silica back out? My knowledge of organic chemistry is... extremely limited. And even if so, how would I do so in such a manner as
to grow a cohesive single crystal?
I also can't tell if the ions responsible for coloring silicon dioxide (iron, in amethyst's case) will carry over in this reaction. Granted, I've no
idea how they'd carry over in water, but hydrothermal amethyst and citrine do indeed exist, so it must be possible somehow.
EDIT: Found this interesting tidbit: http://www.futurity.org/silica-rice-hulls-1080032-2/
This would appear to claim that adding ethanol to the distilled Si(eg) mix from the paper would swap the ethylene glycol for the ethanol, and this
liquid could then be separated and heated to boil off the ethanol and leave behind a crystal of silica. Is this true, or am I reading something
incorrectly here?
[Edited on 1/5/2018 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by elementcollector1  | | Having read that paper, I can't find any references to silica precipitating back out when the reaction mix was cooled. However, ethylene glycol and
silica are two easy-to-find things, so I can try this experiment out rather easily. Unfortunately, I lack any source of TEA - does anyone know of an
OTC product that contains it? |
You might find that other amines would work in a similar manner; you just want one with a low vapour pressure.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
https://hpd.nlm.nih.gov/cgi-bin/household/brands?tbl=chem&am...
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Its on ebay.uk £12/kg including postage
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I did not find that either. What I said was:
"However apparently from the note the reaction produces water and is driven by the water being removed by evaporation as the reaction conditions are
above the boiling point of water.
That suggests there is equilibrium and if the equilibrium is temperature dependent then it may work."
Meaning in a mixture of glycol and water (say 20:1) in a sealed reactor heated to say 200C eventually there will be an equilibrium when the silica is
dissolving (combining with the glycol) at the same rate as the combined glycol/silica is hydrolysed back to glycol and silica. The mixture ratio,
temperature and temperature difference will have to be selected for the best results.
If the equilibrium is temperature dependent then the usual temperature gradient method of crystal growing may work.
It may also just deposit amorphous silica powder.
As already suggested triethanolamine water mixture could also be used and may be more effective or work at a lower temperature.
Triethanolamine complexes with many metals, for example copper and nickel even at high ph so that may be a method of incorporating metals in the
quartz.
I am going to try desolve powdered silica gel (cat litter) and I waiting for a silicon rubber gasket for a stainless steel pressure cooker. Hopfully
the silican rubber should work a few times at up to 200C.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Ah. That makes significantly more sense. I would still like to try the ethanol method, as careful evaporation of the solution seems more likely to
result in crystal formation than a carefully-controlled equilibrium of reactions.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Here is an other note on the subject:
Attachment: Alkoxysilanes laine2015.pdf (625kB)
This file has been downloaded 454 times
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | | Ah. That makes significantly more sense. I would still like to try the ethanol method, as careful evaporation of the solution seems more likely to
result in crystal formation than a carefully-controlled equilibrium of reactions. |
If you are referring to the note you posted how is the ethanol/silica compound decomposed? Can you explain more details about the alcohol method or
have you not got that far.
I would guess that it has to be hydrolysed just like the glycol version. So it has the same problem of shifting the equilibrium in favour of silica
but not so far that a precipitate of silica forms, just far enough that a slightly super saturated solution forms and grows the seed crystal. I
suspect that it will be slow what ever method is used.
The important point for me is can a cheap source of silica be used as an alternative to expensive fumed silica. Ie powdered silica gel from cheap cat
litter.
I am interested in growing quartz if it can be done without too much difficulty but my principal interest is in cheap soluble silica without metal
alkali.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by wg48  |
If you are referring to the note you posted how is the ethanol/silica compound decomposed? Can you explain more details about the alcohol method or
have you not got that far.
I would guess that it has to be hydrolysed just like the glycol version. So it has the same problem of shifting the equilibrium in favour of silica
but not so far that a precipitate of silica forms, just far enough that a slightly super saturated solution forms and grows the seed crystal. I
suspect that it will be slow what ever method is used.
The important point for me is can a cheap source of silica be used as an alternative to expensive fumed silica. Ie powdered silica gel from cheap cat
litter.
I am interested in growing quartz if it can be done without too much difficulty but my principal interest is in cheap soluble silica without metal
alkali.
|
To quote from the article:
"Grain alcohol is then added at the end of the process. It’s chemically similar to antifreeze, so it easily swaps in to replace the antifreeze,
which is then recycled. The liquid silica can then be distilled out of this second solution and used to make a high-purity precipitated silica product
for industrial use."
I'm still not entirely sure what they mean by the 'liquid silica' - presumably it's some kind of ethanol-silica polymer.
The full text referenced in the article can be found here: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/1373...
Reading the article, it seems that adding ethanol to a mix of ethylene glycol causes Si(eg)2 to precipitate, as it is insoluble in ethanol. They note
that it is soluble in methanol, however, and the other literature I found suggests that it can be recrystallized from methanol "with difficulty"
(found in this book).
Though all of these references state that silica can be made from this product, they don't seem to mention how. This abstract notes that pyrolysis of this eventually leads to compounds of the nature MO.SiO2 (where MO is the alkali oxide of the base used),
which is close but not quite what I want.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
To get silica from Si(eg)2 would involve either hydrolysis or pyrolysis, I would guess.
The "liquid silica" mentioned (Si(OEt)4?) might be a possible solvent for growing silica crystals...
[Edited on 6-1-2018 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Having read the first article more thoroughly, I think I understand a bit more now. The reaction of sodium hydroxide, silica and ethylene glycol is
catalytic in nature, in that the base dissolves and reacts to yield sodium glycolato-silicate (SGS), which then aids further dissolution of the
silica. Amorphous silica dissolves more quickly than crystalline silica, and amorphous silica with high surface area (fumed, diatomaceous earth, etc.)
dissolves more quickly than amorphous silica with low surface area (celite, quartz, etc.).
If a small amount of base is initially used, and the dissolution is run for a long time, Si(eg)2 can be recovered dissolved in the excess ethylene
glycol. This can indeed be used to produce Si(OEt)4 or TEOS (tetraethyl orthosilicate) by mixing with ethanol (and possibly a slight amount of acid,
the paper is unclear about this) and distilling off the TEOS at about 170 degrees C.
From there, the TEOS can be either pyrolyzed or hydrolyzed to produce an amorphous and very pure form of silica.
| Quote: | | The "liquid silica" mentioned (Si(OEt)4?) might be a possible solvent for growing silica crystals... |
How so? Would a seed crystal of quartz grow crystalline SiO2 on its surface? Wikipedia lists the decomposition temperature of TEOS as greater than 600
degrees Celsius, which rather defeats the purpose of a low-temperature method (and would likely be hard to control to grow a large crystal).
I was thinking of using a method like the following:
-A seed crystal of quartz is mounted on a rod driven through the center, which is slowly rotated via a motor. This is suspended so that half the seed
crystal is submerged in a heated water bath, while half is exposed to the reaction chamber at any given point.
-While it is rotating, TEOS gas is distilled in at 170 degrees Celsius, reacting with the wetted surface of the quartz seed to grow a new layer of
crystalline quartz in an epitaxial manner (so that the new quartz hopefully follows the crystal template of the old one, making the setup
monocrystalline).
-This continues until the desired size is reached.
I feel like this is the right direction compared to the ultra-high-pressure setup, but there are these last few hurdles to deal with.
[Edited on 1/6/2018 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Here is an interesting graph of the water solublity of quartz and amorphous silica.
Its from this site https://www.researchgate.net/figure/Water-solubility-of-quar...
Unfortuatly I cannot open the document somebody else may be able to.
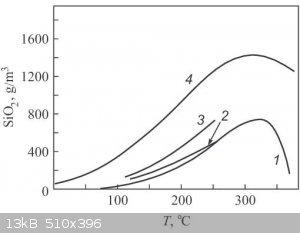
Water solubility of quartz (1), chalcedony (2), cristobalite (3), and amorphous silica (4) according to [2].
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Just tried the first half of the ethylene glycol method. I took a tiny pinch of NaOH, placed it in a test tube, and added about 25 mL of ethylene
glycol (green, concentrated antifreeze). I then heated until all NaOH was dissolved (the test tube's liquid turned slightly yellow, though I couldn't
get a good picture of it).
Next, for my first trial, I added just a pinch of fumed silica, about as much as the NaOH. This dissolved after heating in a stovetop flame for a few
minutes with little trouble, so I added quite a bit more (maybe 5 mL total) and began heating that. However, it started bumping severely, so I've
stopped heating for now and am letting it cool (and hopefully more of the silica will dissolve while it's doing so). So far, not much of the silica
seems to have dissolved, but I'm crossing my fingers.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Raw feedstocks?
Seems to me, the feedstock of choice in the old days, was chunks of dirt-cheap Brazillian Optical type quartz.
The Quartz crystals are dissolved in the lower realms of the reactor, and reformed in the upper realms, onto "seed" plates. Li salts are used in the
solution, to exclude Aluminum from the final crystals. Traces of various elements are incorporated into the new crystals, so that upon
irradiation....Lovely gemstones are produced. If Aluminum isn't somehow excluded from the new crystals, upon irradiation, "Smoky" or Black Quartz is
produced.
Sapphires, Beryls, Topazes, Tourmalines, and Garnets... May likewise be produced.
Got a patent somewhere, I'll see if I can find it.
I'm not seeing a lot of suitable autoclaves out there for sale.
Might be called upon to make your own.
[Edited on 13-1-2018 by zed]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I don’t have suitable equipment to try a serous glycol test yet.
I did try powdering the silica gel with a blender. Very effective compared to PET or aluminium. But Ouch! the air float part of the resulting powder
is nasty, irritating the nose and throat even half an hour later. It has a smell similar to wet clay. Of cause I had very limited exposure. The trick
was to run the blender in bursts so the powder did not heat up too much to limit the thermal currents spreading the air float part of the powder. The
resulting powder still had a gritty component but mostly fine with a very fine component (the air float part)
I did try a NaOH solubility test. I mixed 4g NaOH with 6g of powdered silica gel. I then added 10ml of distilled water with stirring no added heat.
An immediate reaction the solution boiled for a about a minute to produce a clear liquid with the silica gel totally dissolved. Apparently silica a
gel is very reactive compared to fullers earth or fine sand.
Hopefully it will be equally reactive with glycol.
I now suspect that the low temperature hydrolysis of glycol/silica will probably produce hydrated silica gel and that much higher temperatures and
possibly pressure is need for quartz. Apparently some fluorides catalyse the initial products of hydrolysis to react to form an extended network.
That may help to form quartz with a concoction water glycol NaOH and triethanolamine at lower temperatures and pressure.
I still hope to find a phase diagram for various forms of silica that may show that quartz is more stable than silica gel near room temperature. The
reactivity of silica gel compared to quartz suggests quartz may be more stable near room temperature.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I believe silica gel is hydrated, compared to fumed silica which contains much less water. I've no idea how that will affect anything, but found it
worth mentioning.
My idea about getting quartz out of that mess involves a mix of only ethylene glycol, silica, and a little catalytic NaOH, to avoid forming hydrated
silica of any kind. Once I get this distilled into a suitably pure silicon compound, I think I'm going to try something like epitaxial growth on a
quartz crystal substrate, though I'm still not sure how. Hopefully, the existing crystalline quartz template will encourage quartz growth as the
stable phase.
Speaking of which, I just got back from heating it for the second time. A good bit of the fumed silica has disappeared (though whether it's dissolved
or just consolidated, I'm not sure), and the ethylene glycol/NaOH mixture has turned much more yellow in coloration. The bumping stopped about midway
through, so now the glycol appears to be boiling normally, indicating not much solid is in the way of the bubbles. No pictures yet, but I'll try to
get a comparison in here at some point or another. This is definitely easier than building my own 1000 bar pressure vessel...
EDIT: Appears I spoke too soon. Some fumed silica is settling back out of the test tube. It doesn't appear to be as much as I put in, but knowing how
flocculent fumed silica is designed to be, I can't really tell if that means anything.
[Edited on 1/13/2018 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:47 |