NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
What Happens if...
Ok real simple thread, i will start. I will have to add the pics a little later.
Before the rules, let me explain.
My experiment is a longish term one. Ages ago i posted a pic of a bottle containing a few sticks/twig or whatever you call them, of cloves in
Chloroform (I will post it again a bit later).
I forgot about the bottle for months, when i rediscovered it i found it had 4 very distinct layers.
Bottom layer looks very much like Tannin's, but the size of the layer is alot less than the amount of chloroform put in.
Next layer is a kind of light coloured straw layer, it looks about right for the amount of Chloroform put in. An the other layers you will see in a
bit.
So now the task and question.
My what happens if.....
Goes like this
What happens if I put some cloves in a small flask and cover each with one of the following solvents/ Chemicals..
DCM
Toluene
Methanol (very Dry)
methanol 80%/20% water
IPA 70%/30% water
Very Dry IPA
DI Water
Water PH 7
water PH2
Water PH 9
Chloroform Dry
Chloroform and methanol
Acetone
So you ask the question, then go start the experiment. Your allowed to edit and add the first pics in the normal edit time limit. After that the next
post you do is an update if its a long experiment or the final result.
Ideally you shouldnt know the answer before you start. Your not allowed to ask questions about it, but others can make guesses.
In my case I dont know what is going to happen except the chloroform one. Once complete I will TLC each layer from each flask and post the pics of
what each extracts.
The what ifs can be as simple as you like, but you got to actually do it to find out. So if your a muppet and post.....
What If I drink 3 ltr 98% H2SO4, then you do it and post the pics.
in other words, stupid is ok as long as you actually do it!
Simple ones are good as well.
Back soon with the pics, results will come when something happens.
These pics still dont show the 4 layers properly! plus moving them into the warm started one layer to cloud a little
Got the flasks ready (50ml) Started weighing and recording fresh cloves, put some labels on and then ran into a problem.
I dont have enough stoppers of that size!! So I have halted and have cast some molds, in the morning i will pour some chem resistant silicon and wrap
a little ptfe tape around them. They should do fine as stoppers!
I would do that tonight but need the VAC pump in kitchen and its loud!
Should still be within the time edit and rules though
[Edited on 13-1-2018 by NEMO-Chemistry]
![20180112_235045[1].png - 263kB 20180112_235045[1].png - 263kB](https://www.sciencemadness.org/whisper/files.php?pid=503626&aid=64037)
  
[Edited on 13-1-2018 by NEMO-Chemistry]
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
Cloves, eh?
What happens if...
Ground cloves in uncle Jimmy's high-test moonshine with a pinch of PdCl2 and a dash of CuCl2 for seasoning.
Place in a red Solo cup, drop in an aquarium air stone (on an aquarium air pump), aerate for a day in a sunny spot in late August while playing "Rusty
Cage" by Johnny Cash.
Since it's an oxidation, I like to think it can be legitimately described as wacking off.
Some day... :-)
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Reboot  | Cloves, eh?
What happens if...
Ground cloves in uncle Jimmy's high-test moonshine with a pinch of PdCl2 and a dash of CuCl2 for seasoning.
Place in a red Solo cup, drop in an aquarium air stone (on an aquarium air pump), aerate for a day in a sunny spot in late August while playing "Rusty
Cage" by Johnny Cash.
Since it's an oxidation, I like to think it can be legitimately described as wacking off.
Some day... :-) |
Ok you asked the question, so now go do the experiment and post the pics. You can leave the wacking off pics out though.
The pics are not good of the first bottle, but in person when you see it, its a really interesting effect and counter intuitive. It was Chloroform, so
the main layer should be on the bottom, but it isnt.
So what in cloves dissolves in Chloroform and is dense enough to form an extremely distinct layer. The shiny bit of the bottom layer shows just how
much the layer wont mix.
Its like a molecule deep gap! then you get more chloroform with a oil dissolved in it. The point was the chloroform was dry and freshly made, the
cloves made have had some water in, but this would be tiny. So 4 separations of a solvent from a single plant material is pretty interesting.
So I want to know which of the many components of cloves are separated into layers with different solvents, and do any other solvents form 4 layers.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have annotated the first pic
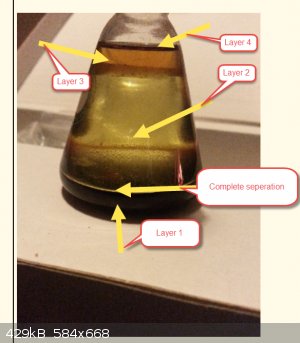
I thought you were taking the piss, it went way over my head. But turns out thats really interesting chemistry. not sure why the chlorides are there
though??
[Edited on 13-1-2018 by NEMO-Chemistry]
[Edited on 13-1-2018 by NEMO-Chemistry]
Attachment: oxidation eugenoletc.pdf (242kB)
This file has been downloaded 298 times
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
https://www.youtube.com/watch?v=XYkC6ocDdR4
https://en.wikipedia.org/wiki/Wacker_process
Kind of a classic. First developed by the petrochem industry to create aldehydes from alkenes, but later extended to ketone production.
As far as I know the catalyst mix doesn't need to be chlorides specifically; I've seen acetate versions.
For me the neat thing is that, in spite of the reaction being driven by oxygen gas, it's actually water being added to the alkene; the oxygen is just
oxidizing the catalyst.
Traditionally the reaction is run under pressurized oxygen, but...I expect that air alone at ambient pressure can do it since it's simple oxidation,
not like the catalytic hydrogenations that require very high gas pressures (I assume to crowd hydrogen atoms onto the catalyst surface in an unstable
concentration.)
If I had to take a guess at your layers, I'm thinking (from the bottom): Silt/sludge from the plant material with water clinging to it; chloroform
layer; aqueous layer; lower density fats from the cloves that managed to migrate to the top over time.
[Edited on 14-1-2018 by Reboot]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Got the pics! Cant upload yet as i need to mess with them (taken on camera), the post above is really interesting and seems spot on, except for one
thing.
While the bottom layer does have the crap in, there is also a liquid. You cant see it in the pic, but if you look at it and didnt know better, you
would swear this was Bromine!!
You know how when you make Bromine it kinda swirls around the bottom, and it looks like every other molecule is jumping out of its way? Well the
bottom layer behaves just like that, hence the very defined separation of the bottom layer. Its a really strange dark brown reddish liquid, almost
same color of Bromine but more Brown than Bromine is.
The Acetone one I will take a pic of in a couple of days, it looks like it extracts but dosnt separate anything!
|
|
|