| Pages:
1
2 |
godchem
Harmless

Posts: 4
Registered: 17-2-2007
Member Is Offline
Mood: No Mood
|
|
Picric acid - with phenol and HNO3
Anybody knows how can we make Picric acid with only ccHNO3 and phenol? I heard it's possible, but I don't know this process. THX!
Probably this method is much cheaper, than phenol + ccH2SO4 + ccHNO3 method.
\"When I was young I played with legos, but now I am older and I play with atoms. - Alexander Shulgin\"
godchem
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
the H2SO4 is there for a reason, the reason is that the nitration produces water and the water slows down the nitration of further phenol by diluting
the nitric acid. Off the top of my head I suspect you would stop at 2,4-dinitrophenol or a nixture of that plus o-and p-nitrophenols if you omit the
sulfuric acid, which removes the water.
Making picric acid produces quite a bit of tar from which you need to seperate the product.
I haven't nitrated phenol since I was a student when it was a standard instructional lab experiment.
I have read of some modern experiments on this, I think with mixed phase systems, or transition metan salts for catalysis. But I don't remember the
details.
|
|
|
h0lx
Hazard to Self
 
Posts: 69
Registered: 31-12-2005
Member Is Offline
|
|
Wasn't sulfuric used for creating phenol sulfoesters, which are easier to nitrate?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Not at all. Phenol is extremely easy to nitrate because the hydroxyl group is an activating one.
The point of the sulfuric acid is to keep the nitric acid from dropping below the concentration at which the nitronium ion forms. It does this by
absorbing the water of the reaction as it is formed.
I think you had better have a look at a few good books on aromatic nitration.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
The point of the sulfuric acid is to keep the nitric acid from dropping below the concentration at which the nitronium ion forms. It does this by
absorbing the water of the reaction as it is formed.
|
Is water also formed in a PETN synthesis using the HNO3/PE method? If so, should one use H2SO4 in that synthesis as well? Actually, if water is always
produced during a nitration, shouldn't H2SO4 be used in any synthesis?
I'm not a chemist so my apologies if that's a dumb question.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If you write out the equation for reaction between HNO3 and pentaerithritol you will see that when one mol PE is nitrated four mols H2O are formed.
When one mol of glycerin is nitrated three mols H2O are formed.
When one mol phenol is nitrated to trinitrophenol three mols water are formed.
The same with TNT, and so on.
In every one of these cases we use the mixture of equal parts cocentrated HNO2 abd concn H2S)4. In some instances we are obliged in the final stage of
an aromatic nitration to employ stronger nitric acid and stronger sulfuric acid (oleum, H2SO4 plus SO3).
The precise mechanism of nitration of alcohols to nitro esters as opposed to aromatic nitration (replacing an H on benzene ring) differ but the
practice of employing a dehydrating agent (usually H2SO4) along with HNO3 is almost universal.
An exception is the Bachman process for RDX where acetic anhydride takes the place of H2SO4 and participates in the rxn in other ways, but that is a
nitramine and not a nitro-ester nor an aromatic nitro compound.
There are people on this forum who can tell you a lot more about nitration than I can, I am just kibbitzing.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
In every one of these cases we use the mixture of equal parts cocentrated HNO2 abd concn H2S)4.
|
I'm not a hundred percent certain of this but I don't believe I have ever stumbled upon a PETN synthesis that used H2SO4.(well except of course when
you use KNO3/H2SO4 instead of HNO3)
So far I've only used HNO3/PE to make PETN and my yields are never impressive. I always blaimed my yellow HNO3 for that. You think adding an equal
amount of Liquid Fire as I have of HNO3 should improve my yields then?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
What does the Davis book say for starters? Mixed acid I bet, but it's been twenty years - maybe thirty since I looked between those covers. For
something more au current I can peek in PATR 2700.
H2SO4/KNO3 gives you mixed acids with K sulfate byproduct.
PE is a polyol just like glycerine. How do you nitrate glycerine? Mixed acids. So without looking it up I would say yes, mixed acids would have to
work better than HNO3 alone. Everything else being equal. Why would you not want to employ sulfuric? Few things are as cheap as sulfuric.
Well shiver me timbers, you are right according to Davis, p.279. Naoum's procedure calls for 400 cc fuming nitric acid (d.1.52) to 100 g PE. Note that
this is c.680 g of acid. Sulfuric acid is only added at the end to complete precipitation of the PETN.
The improved procedure also given employs white fuming nitric acid (ordinary fuming acid decolorized with a little urea to destroy HNO2) and no
sulfuric acid. Again 400 cc acid to 100 g PE. However this time the mixture is quenched with ice water and worked up from there. See the attachment
for details.
I am frankly perplexed as to why H2SO4 is eschewed in the case of PETN and it is certainly the norm with NG. And certainly the norm with picric acid,
and TNT, etc. There ought to be an explanation for this and I am going to find it I bet in Federoff or one of the other good books on this yopic.
Fuming nitric acid is relatively expensive and probably more so than ordinary nitric acid plus sulfuric acid combined. I know the tricks for making
RFNA from ordinary nitric, and for making white FNA from red. And of course the really fun distillation of 100% nitric from nitric and sulfuric acids.
What joy.
PE is c.136 g/mol. I'd have to look up the molarity of HNO3 d.1.52 but just as a guess, let's assume 95% acid and we have 680 g of it. So there's a
good 10 mols there, a >3:1 molar excess.
Why wouldn't a smaller excess of HNO3 of same density work just as well in presence of conc H2SO4 in similar mass?
Does the sulfuric acid chew up the PE? It certainly chews up sucrose. That could be the reason. But it does not east glycerol. Oh well. I guess I will
read a book and learn something. Not too old for that.
[Edited on 17-2-2007 by Sauron]
[Edited on 17-2-2007 by Sauron]
Attachment: davis279.pdf (173kB)
This file has been downloaded 928 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Sauron
H2SO4/KNO3 gives you mixed acids with K sulfate byproduct.
|
Actually the byproduct is the acid sulfate , the bisulfate ,
not the sulfate .
Can't figure why anyone would want to use expensive phenol as the precursor for picric acid anyway .....
instead of aspirin , or other less expensive precursors than phenol .
Why use phenol ????
You by inference declare you are worried about the cost of H2SO4 .....when choosing phenol as the precursor for
picric acid ? That doesn't make any damn sense .
Anyway , IIRC there is a very specialized way of using straight nitric for the nitration ....IIRC it is possible by
means of a special technique , maybe a nitrosation
intermediate ....I don't recall exactly what was the
method . Never really paid too much attention because
phenol is definitely not your precursor of choice for
picric acid . If you are going to use phenol as the precursor
then it stretches credulity to think about the concern over economics being adversely affected by the cost of H2SO4 .
[Edited on 17-2-2007 by Rosco Bodine]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hello, I think I passed a cume this morning . .
I was always under the impression that in the case of nitration with mixed acid, the nitric acid was protonated by the sulfuric acid to yield the
nitronium cation which acts as an electrophile that the ring can attack (see attached).
Cheers,
O3
[Edited on 17-2-2007 by Ozone]
[Edited on 17-2-2007 by Ozone]
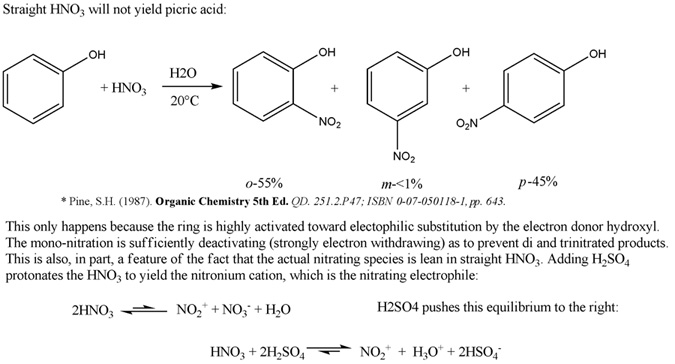
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Quite right about the bisulfate of course. The phenol was the other fellow choice not mine. My recollection is that the industrial route was vis TNCB
with the -OH only going on last, if my meory isn't failing me.
I'm not sure why anyone wants picris at this point anyway unless he needs an electrophoresis reagent, which seems farfetched. As exlosives go it was
passe sometime prior to Prohibition. Shock sensitive salts, cavities in castings, all sorts of technological hassles of that sort. Still lives on in
the form of the styphnates of course, if you count resorcinol as close enough kin to say that.
While we have your attention, @Rosco, please have a look at my last post above as I am sure you can enlighten me as to just why mixed acid is not
employed in nitration of PE? It seems an anomaly.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Not everyone finds phenol expensive, Rosco. Especially not the industry, which prepares your salicylic acid and ASA from phenol.
Also, phenol as the precursor for picric acid gives very good yields, over 90%, and when looking at the molar weights of phenol and pircic acid, you
see that you get a much greater weight of picric acid that what you used in phenol.
EDIT: H2SO4 is used in the nitration beacuse phenol must first be sulfonated to the 2,4- disulfonic acid before swapping the sulfonic acid against
nitro groups and introducing the third one.
[Edited on 17-2-2007 by garage chemist]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Congrats in advance, @O3, I' confident you did fine.
I think the nitronium ion is around just fine as long as the concentration is sufficiently high, I recently saw the magic number but will have to find
it again before I commit and make a fool of myself.
If H2SO4 were needed to protonate HNO3 to up the population of nitronium ions then why is the HNO3-alone nitration of pentaerythritol efficient? No
sulfuric in there at all in one version and only at the end in the other.
I agree completely that in almost every other nitration I can think of mixed acid is the norm. So why not in case of PE and if your mechanism is
right, why does it work and work well (85-90%)?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
See? I said way up the thread that there were plenty guys here who know a lot more than I do on the subject of nitration, and now they are here, so I
can sit back and shut up. 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@ Ozone . There is more than one way to skin a cat ,
and there are ways of moderating aromatic nitrations
so that no sulfuric acid is required and indeed the nitration is accomplished by nitric acid , perhaps indirectly
by way of some of its thermal decomposition products ,
generating nitroso intermediates . I am pretty sure I have seen this reported for not only picric acid , but styphnic acid and also TNT .....in "
special exception " nitration conditions which use no sulfuric acid , no phosphoric acid , no acetic anhydride , ect. .....but
use only nitric acid .
@Sauron
The preferred method for PETN uses straight nitric of
~97% concentration , and it is a very efficient nitration
98% yield based on PE , even in the absence of H2SO4 . And indeed PETN is anomalous in regards to this ease of nitration compared with other
precursors for nitration . PETN is one of the most economic nitrations in terms of acid utilization .
Also , there is nothing anachronistic about picric acid
and its derivatives which are still unique in their economy/performance/versatility/technical simplicity .
If there is a kind of "holy grail" explosive material ....
picric acid in one form or derivative or another , does it all , and no other material does so many things so well .
[Edited on 17-2-2007 by Rosco Bodine]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
And stains the hell out of your proteins!
Presulfonation is definitely possible, and many mechanisms involve this as a step (as you said). I've always seen it as the ring attacking nitronium.
This is particularly applicable with phenol (and likely, aniline) where ring activation can lead to sulfonation without the use of oleum (which is
what we have to use otherwise).
I've got the ACS monograph "Industrial Nitrations" which describes many interesting and, in many cases, "mild" and/or specific nitration
reagents/conditions, if anyone's interested.
Anyhoo, a mixed acid method (wherein the equation uses the sulfonate as an intermediate) is given here:
http://www.powerlabs.org/chemlabs/picric.htm
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
h0lx
Hazard to Self
 
Posts: 69
Registered: 31-12-2005
Member Is Offline
|
|
| Quote: | Originally posted by Rosco Bodine
Can't figure why anyone would want to use expensive phenol as the precursor for picric acid anyway .....
instead of aspirin , or other less expensive precursors than phenol .
Why use phenol ????
|
Because pure phenol is cheaper for some people than aspirin by the gram, and ASA pills are also contaminated by binders?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
So use benzene and mercuric nitrate complexed oxynitration ,
or buy a pound of pure veterinary aspirin ,
or sulfanilic acid .....
any of the three should be cheaper than phenol .
|
|
|
h0lx
Hazard to Self
 
Posts: 69
Registered: 31-12-2005
Member Is Offline
|
|
Well phenol is cheap enough for me, and I never succeeded in the ASA route, either got a runaway or too low of a yield to bother xalising out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Well then obviously you didn't use Rosco's good old country recipe 
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
After reviewing the basics (Davis) it appears that @Rosco (unsuepeisaingly to me) is absolutely right and phenol is a terrible feedstock to picric
acid. The French 4-stage process from phenol mostly was good for making DNP which they preferred to admix with picric acid anyway.
@Roscor already mentioned the catalytic process from benzene using mercuric nitrate, this was already being done on plant scale at time of the Davis
book.
One that @Rosco did not mention but I'm sure he is well aware of is what I alluded to earlier, nitration of chlorobenzene to DNCB and then converting
it to picric acid. DNCB is also able to be feedstock for a number of other useful military explosives and bears close consideration.
If you nitrate phenol directly, you get losses to tars, losses to oxidation (oxalic acid), losses to red fumes, and poor yields of the intermediates
and picric acid.
If you sulfonate phenol things are a bit better but more laborious and have no advantage over either the synthetic (chlorobenzene) or catalytic
(mercuric nitrate/benzene) routes.
So all in all I would say listen to @Rosco when he deigns to speak.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Ah yes, another way of skinning the cat involves the use of nitronium salts (think NO2+NO3-) although in practice the fluoroborate appears to be the
best route. Take note that these nitrations take place without H2SO4 or dehydrating reagents.
This morning I was reading up on yields for various trinitrated species and had also drawn the conclusion that trinitrochlorobenzene was an attractive
target (for one, the feed is cheap). I'll use this, then as a model for how a nitronium salt nitration would go:
Quoting Albright, L.F. and Hanson, C. (1975). Industrial and Laboratory Nitrations. ACS Symposium Series 22, pp. 1-47.
"Nitronium tetrafluoroborate is most conveniently prepared by adding anhydrous HF to nitric acid in a solvent such as nitromethane, methylene
chloride, etc, and then saturating the solution with BF3.
HNO3 + HF + 2BF3 = NO2+BF4- + BF3.H2O
A nearly quantitative yield of the stable nitronium salt can be obtained. An undergraduate in an afternoon's work, can prepare up to a pound of the
salt...". "Nitronum salts can be stored at room temperature indefinitely without decomposition...all nitronium slats are..very hygroscopic and must be
stored and handled with precautions to avoid moisture.
The data for chlorobenzene going to TNCB is given stepwise:
Chlorobenzene-->o,p-chloronitrobenzene 10°C, 10 min, 92%.
o-nitrochlorbenzene-->2,4-dinitrochlorobenzene 30°C, 20 hr, 77%.
2,4-dinitrochlorobenzene-->picryl chloride 100°C, 10hr, 80%.
The added advantage of this method is that aromatic esters (and apparetnly nitriles, as well) can be nitrated without cleaving the ester, viz methyl
benzoate-->m-nitromethyl benzoate 30°C, 20 min, 88%.
The given %s above are yields.
Ack. Rats! All of the oxidations listed took place in tetramethylene sulfone and a substrate to salt ratio of 1:1.25 (mono) and 1:2 (dinitro) EXCEPT
2,4-dinitrofluoro and 2,4-dinitrochlorobenzenes which were carried out in 100% H2SO4 (apparently these need a little kick to yield trinitro species).
Anyhoo, a nice paper desctribing the use of various "exotic" nitrations including the tetrafluoroborate salt is attached for your perusal.
Cheers,
O3
Attachment: Friedel Crafts-type nitrations_01.pdf (1.1MB)
This file has been downloaded 1172 times
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Last time I looked nitronium salts cost a fortune and were not fun to make.
By comparison, N205 in situ is a walk in the park.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
How about mixing a bit of sugar or ethanol with mildly concentrated HNO3 , and dripping in the phenol slowly over about 10 hours at a mildly warm
temperature like 35C to perhaps get a nitrosophenol intermediate ,
boil that down or evaporate and concentrate it and
nitrate the nitrosophenol with a more concentrated
HNO3 and higher temperature .
Never heard of it .....but it would seem possible .
Of course the sulfonation is still very likely a better way to go .....but the interest here seems to be for whatever
reason ....a " no sulfuric acid method " . Go figure .
BTW ......
Wasn't the original poster of this topic the same who was saying in the other thread about urea and sodium nitrate being heated together to make
sodium azide ?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If he can make NaN3 that way he can make Pb into Au.
Isn't formaldehyde the usual way to up the HNO2 (NO) content of HNO3? In other works, make RFNA out of ordinary conc nitric?
Can you just keep feeding in formalin till you get desired density or is there a point of diminishing returns when you must resort to distillation?
|
|
|
| Pages:
1
2 |