chemtag
Harmless

Posts: 5
Registered: 28-12-2017
Member Is Offline
Mood: No Mood
|
|
Reaction evolving H2 or CO gas, need help identifying
In a reaction of carbon black with hydrogen peroxide and nitric acid (all at ~75 C) I was able to detect (qualitatively) either CO gas or H2 gas. I
don't know which one because I am using a gas detector intended for confined space entry that has a CO, O2, and H2S detector. The meter reads a large
amount of CO (the reader maxes out at 1000 ppm, and it quickly saturates that). However, the detector has a cross-sensitivity with H2, meaning H2 gas
would give a positive reading on the CO detector.
The reaction is a bit of a witch's brew, but there is a strong oxidizer (H2O2) in the presence of carbon, so you could imagine some sort of partially
oxidized carbon forming CO gas. On the other hand, H2O2 can react as a reductant under certain conditions, so you could imagine some of the H2O2
reducing the H+ ions from the nitric acid to make H2 gas.
The evolution of either gas (H2 or CO) at these volumes is a potential problem as scale up would have to address either one's safety hazard. However,
it would still be nice to nail down which one is evolving, and I am not sure the best way to do that. I am also not really sure which one is more
likely to be produced under these reaction conditions.
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
CO or CO2. Hydrogen surely isn't.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Certainly no H2 is formed under such conditions (hot, strongly oxidizing). Reduction of H(+) ions by H2O2 is something which definitely will not
happen.
Production of CO could be possible, but it surprises me somewhat. I, however, do not want to rule out that possibility, given your observation of your
CO-detectormaxing out on the gas mix.
|
|
|
chemtag
Harmless

Posts: 5
Registered: 28-12-2017
Member Is Offline
Mood: No Mood
|
|
I am in agreement that reduction seems very unlikely. One of the engineers here thought that the CO could be the reducing agent to form H2 and that a
mixture is forming. Again, I am having a hard time wrapping my head around the likely hood of that but am kinda falling back on what Woelen said (a
hot mixture of HNO3 and H2O2 is going to be powerfully oxidizing, and reductions shouldnt happen).
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Not to be a wise-ass...but if something is oxidized, something else must be reduced...those electrons have to go somewhere!
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Hydrogen Peroxide gets reduced to water and oxygen.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
[brainfart]
Bubble it through a cold CaOH solution scrubber and then through an efficient warm NaOH solution scrubber. CO2 will be caught in the first flask,
quantity can be determined by drying the precipitate. CO will be caught in the NaOH, and can be determined by formate titration. The rest is hydrogen.
[/brainfart)
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Can CO really be converted to formiate by simply bubbling it through a solution of NaOH? I think you need a little more for that.
I understood that it only can be done at elevated temperatures (around 150 C or so), under high pressure and with suitable catalysts. CO is fairly
inert at room temperature.
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
It can't, just like it can't with water to form hydrogen and carbon dioxide. If it did, it would be easy/easier to produce hydrogen from coal at home.
Sodium formate is prepared by HEATING sodium hydroxide with carbon monoxide UNDER PRESSURE:
NaOH + CO → HCOONa
Reaction with steam catalyzed by ZnO–CuO or Fe–Cr yields hydrogen. This reaction, known as “water gas shift,” is a source of industrial
hydrogen.

Carbon monoxide thermally decomposes to carbon and CO2 when heated from 500 to 700 °C; while catalytic decomposition occurs at ambient temperatures
in presence of Pd/silica gel or MnO2/CuO catalysts.
(Handbook of Inorganic Chemicals)
[Edited on 16-2-2018 by RawWork]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
"The Removal of Carbon Monoxide from Air", Lamb, Bray & Frazer 1920.
https://pubs.acs.org/doi/abs/10.1021/ie50123a007
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
If CO would produce formates with alkalis in water, formic acid would be called carbonous acid and CO carbonous anhydride (analogous to phosphorous
acid). But it is not called that, because this reaction does not work.
Smells like ammonia....
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Ic or ate means higher or highest most common oxidation state. Ous or ite means lower or lowest most common oxidation state. But it's only when
element is in combination with oxygen (and hydrogen in case of acid salts, also called bi) and it is anion in that combination. For example:
sulfur oxidation state +6 is sulfate (sulfuric)
sulfur oxidation state +4 is sulfite (sulfurous)
carbon oxidation state +4 is carbonate (carbonic)
carbon oxidation state +2 is carbonite (carbonous) (maybe not officially because it's organic chemistry)
But formate is anion that contains hydrogen too (which in inorganic chemistry would add bi to it's name). And oxidation state is +4 which would be
more likely carbonate. But because it's organic and covalent, we can stop here. There exists acetic acid and anhydride, and many compounds that
contain same number of atoms, but are neither carbonate nor carbonite. That is organic chemistry, more complex than inorganic.
Read IUPAC nomenclature (red book and blue book). Or Golden book of chemistry experiments. Or this:
https://en.wikipedia.org/wiki/IUPAC_nomenclature_of_inorgani...
Formic acid:
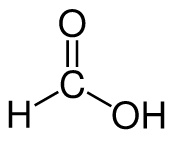
Formate:
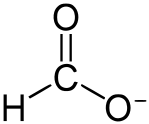
[Edited on 16-2-2018 by RawWork]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
For a simple qualitative test, collect some of the gas in an inverted test tube. If hydrogen is present in significant quantities, when a flame is
brought to the opening, it will ignite with a high pitch popping sound (giving the name to the ‘pop test’) which is similar to when you pop your
cheek with your index finger.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
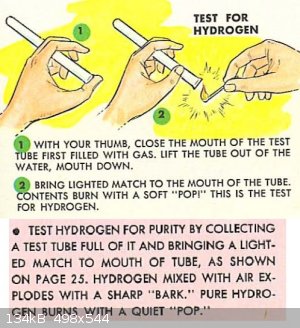
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
When thine retort dost bubble, and that rightly,
Take thee a moment to Observe, lest the fuge take hold.
Thence take a firey brand, yet not aflame.
With great fortitude put it forth into the Mouth of the Dragon.
If seest thou the brand reflamed, yea has thou seen the spirit of the Bringer of Water.
If the brand stayeth unfired, come next to the Proof of the Darkness.
Place the open Mouth of the Dragon into a vessel filled with the pure and clear Milk of Lime.
If the clear milk is thereby turned to white, then thy Dragon emits the vapours of the Dark Matter.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chemtag  | In a reaction of carbon black with hydrogen peroxide and nitric acid (all at ~75 C) I was able to detect (qualitatively) either CO gas or H2 gas. I
don't know which one because I am using a gas detector intended for confined space entry that has a CO, O2, and H2S detector. The meter reads a large
amount of CO (the reader maxes out at 1000 ppm, and it quickly saturates that). However, the detector has a cross-sensitivity with H2, meaning H2 gas
would give a positive reading on the CO detector.
The reaction is a bit of a witch's brew, but there is a strong oxidizer (H2O2) in the presence of carbon, so you could imagine some sort of partially
oxidized carbon forming CO gas. On the other hand, H2O2 can react as a reductant under certain conditions, so you could imagine some of the H2O2
reducing the H+ ions from the nitric acid to make H2 gas.
The evolution of either gas (H2 or CO) at these volumes is a potential problem as scale up would have to address either one's safety hazard. However,
it would still be nice to nail down which one is evolving, and I am not sure the best way to do that. I am also not really sure which one is more
likely to be produced under these reaction conditions. |
The carbon black may be a source of dust particles containing transition metals. The reaction of the latter with H2O2 in the presence of H+ (from
HNO3) may produce some hydroxyl radicals (.OH) via a fenton reaction. The reaction of the latter radical with any formed CO could result in some H2.
Reactions:
Fe(2+) + H2O2 --Acid-> Fe(3+) + .OH + OH- (fenton reaction)
Fe(3+) + H2O2 --> Fe(2+) + .HO2 + H+ (recycling ferric see, for example, http://lib3.dss.go.th/fulltext/Journal/Environ%20Sci.%20Tech... )
C + H2O2 --Fe(2+)--> CO + H2O (see https://www.sciencedirect.com/science/article/pii/0016236194... )
CO + .OH = CO2 + .H (see https://www.sciencedirect.com/science/article/pii/S008207847... )
.H + .H = H2
So, in my opinion, both CO and H2 could indeed be formed here.
[Edited on 21-2-2018 by AJKOER]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by aga  | When thine retort dost bubble, and that rightly,
Take thee a moment to Observe, lest the fuge take hold.
Thence take a firey brand, yet not aflame.
With great fortitude put it forth into the Mouth of the Dragon.
If seest thou the brand reflamed, yea has thou seen the spirit of the Bringer of Water.
If the brand stayeth unfired, come next to the Proof of the Darkness.
Place the open Mouth of the Dragon into a vessel filled with the pure and clear Milk of Lime.
If the clear milk is thereby turned to white, then thy Dragon emits the vapours of the Dark Matter. |
Are these the tests for oxygen and carbon dioxide by any chance? A smouldering splint will reignite in a high oxygen environment, and bubbling CO2
through calcium hydroxide solutions produces a milky calcium carbonate precipitate.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yea, verily.
Edit:
Bugger. Just looked it up.
Alchemists called oxygen the 'Bringer of Acid/Sourness', not the 'Bringer of Water'.
[Edited on 21-2-2018 by aga]
|
|
|
chornedsnorkack
National Hazard
   
Posts: 521
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LearnedAmateur  | | For a simple qualitative test, collect some of the gas in an inverted test tube. If hydrogen is present in significant quantities, when a flame is
brought to the opening, it will ignite with a high pitch popping sound (giving the name to the ‘pop test’) which is similar to when you pop your
cheek with your index finger. |
How is that sensitive specifically to hydrogen, rather than any other flammable gas/vapour?
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
Quote: Originally posted by chornedsnorkack  | Quote: Originally posted by LearnedAmateur  | | For a simple qualitative test, collect some of the gas in an inverted test tube. If hydrogen is present in significant quantities, when a flame is
brought to the opening, it will ignite with a high pitch popping sound (giving the name to the ‘pop test’) which is similar to when you pop your
cheek with your index finger. |
How is that sensitive specifically to hydrogen, rather than any other flammable gas/vapour? |
That is what I was thinking. Carbon monoxide is highly flammable too right? That way the test would not be able to distinguish between hydrogen or
carbon monoxide as far as I am concerned...
Yep, I have a chemistry blog!
18thtimelucky.wordpress.com
"Amateur chemistry does seem like being in a relationship with someone very beautiful and seductive but has expensive taste, farts a lot and doesn't
clean up after themselves, but you love them anyway" - a dear friend |
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Burn rate would be one indicator, for example if you had hydrocarbon/alcohol vapours then these would result in a flame and be more visible - if CO
reacts in a similar way with a flame, it would likely be lower in pitch if it even ‘explodes’ at all rather than just burning. You could also coat
the inside of the test tube with calcium hydroxide solution - when CO burns, the CO2 will turn the solution white, whereas hydrogen just produces
water thus a negative result. Two different chemicals with similar properties are extremely unlikely to share exact characteristics under certain
conditions, and to keen senses, it can be a reliable way to correctly identify something which is one reason why the pop test is so useful, since not
many gases behave like hydrogen does, especially the more common ones.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
Pitch change did cross my mind but I assumed it would still be quite hard to tell, especially when there might possibly be a mixture of the two.
The colour of the flame is a good idea though. The hydrogen would be colourless/slightly orange and then the carbon monoxide blue?
Yep, I have a chemistry blog!
18thtimelucky.wordpress.com
"Amateur chemistry does seem like being in a relationship with someone very beautiful and seductive but has expensive taste, farts a lot and doesn't
clean up after themselves, but you love them anyway" - a dear friend |
|
|
chornedsnorkack
National Hazard
   
Posts: 521
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
To think more of the pop test, I think all flammable gases contain hydrogen, carbon or both. Correct?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
When other proofs fail, proceed to The Proof Of Gas.
Bringest thou a lighted brand and thrust it forth into the vapours, yet confined.
If a Loud Trump is heard, yea the spirit of the Bringer of Water is present.
[Edited on 1-3-2018 by aga]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I think you are all out to lunch! Woelen I suspect is closest. It is very unlikely that you are going to get any reaction between carbon black and
either nitric acid (unless it is >90%) or hydrogen peroxide. I don't know what you re trying to achieve but I suggest you check out some of the
threads on SM on graphene oxide and the preparation of mellitic acid acid. The best yield of the latter I could get was very poor from days of
refluxing. Hydrogen peroxide is without effect and because it is at best a 30% solution you are simply adding a lot of water to the nitric acid.
However, there are two points to consider:
1) hydrogen peroxide can also act as reducing agent so are you get nitrogen oxides produced by the reduction of nitric acid?
2) As a mining engineer I am very familiar with the type of instrument you are using and I can tell you for certain that the results you are getting
mean nothing at all! These instruments are designed and calibrated for a certain set of circumstances and make assumptions about what types of gas or
volatiles that may be around in that particular environment. For the type of instrument used by civil engineers for confined space work outside of the
petrochemical industry the sensors you have will be screwed up by volatile hydrocarbons and nitrogen oxides. You will need to read the manufacturers
manual very carefully and also have a copy of the calibration certificate. With nitrogen oxides sensors you are usually looking at ppm ranges from
vehicle exhaust in underground operations not % ranges. At these concentration there is likely to be a lot of interference with the other sensors.
|
|
|