MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
CO2 Solubility vs Partial Pressure
My video on the Generon nitrogen membrane filter received some good comments, and one of them sent me on a path of calculation that I'm having a bit
of trouble on. I wrote up my findings on my blog, here: http://thehomescientist.blogspot.com/2018/03/generon-filter-...
But I'll give a summary here. I'm burning ethanol in a closed container to use up the oxygen, as a way of measuring the percent of oxygen in the gas
in the container. It was pointed out that ethanol produces 2 moles of CO2 for every 3 moles of O2, which in retrospect is obvious:
C2H5OH + 3O2 == 2CO2 + 3H2O
My goal is to find out the solubility of carbon dioxide in water, to determine if this affected my results or if it all just dissolved. I found a
chart on CO2 solubility here:
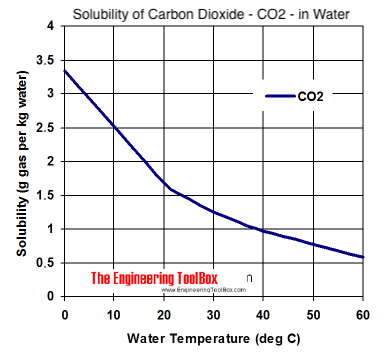
And in my linked post I took the 1.5g/kg at ~25C value and called it a day. However the site also mentions "Note that for gases in combination
with other gases - like oxygen in air - the partial pressure of the gas must be used. Example - in air with normal composition oxygen counts for
approximate 20% of the total pressure."
I tried finding a chart of solubility that also included pressure, but came up dry. Can anyone point me to a reference? It would have to be for fairly
low partial pressures; in one experiment I produced 88.2mL of the gas in a 630mL volume, yielding a partial pressure of 14 kPa.
Or is there a way to calculate solubility based on the pure gas chart above? Surely it's not as simple as taking a % of the charted values?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
https://chemengineering.wikispaces.com/Henry%27s+law
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Knowing next to nothing, my first instinct is to ask if burning it in ethanol is the simplest method of ascertaining the O2 concentration.
Mumble mumble.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
| Quote: | | At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure
of that gas in equilibrium with that liquid. |
Huh, so it really is that simple? That graph above is for atmospheric pressure (101 kPa), so it would be
(1.5 g/kg) / (101kPa) = (x) / (14kPa)
x = 0.21 g/kg
Is that correct?
Quote: Originally posted by aga  | Knowing next to nothing, my first instinct is to ask if burning it in ethanol is the simplest method of ascertaining the O2 concentration.
Mumble mumble. |
It seemed simple at the time. The other option was to rust fine steel wool in the container, but that takes a very long time. I tried it with a hand
warmer pack, since that contains iron powder and works by rusting quickly, but that didn't give very good results over a 24 hour period. I had to
abandon it because I needed to return the filter. Perhaps I should have planned better...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I used Henry's Law to calculate the the concentration of CO2 in the water assuming the following partial pressure over the water:
P(CO)2 = [(82.5mL)/(630 mL)]101.325 kPa = 13.27 kPa
29.4L*101.325kPa = Henry's Law constant for CO2 in water at 25°C.
from Henry's Law: C(CO2) = P(CO2) *mole/(29.4 L*101.325kPa) =
= (0.0045*mole)*(44g/mole)(L/Kg) = 0.196g CO2/kg water.
This is very close to your value.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|