| Pages:
1
..
9
10
11
12
13
14 |
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
No, a manifold is a pipe with multiple connection to other pipes, for the purpose of distributing to those other pipes or collecting from them. Often
the manifold is larger than the feeder pipe, because it handles a larger flow.
So one largish pipe from the boiling has a number of small tube connected to it. The smaller tubes are in some sort of an array, their bodies in
parallel and adjacent but separated a bit. Their other ends all connect to a second manifold that combines the flows and takes it to the quencher.
The second, hot side, manifold would likely be smaller than the boiler side one, because you want to minimise the time the gases remain hot, and thus
want high flow rates.
Read the Project00 file - for larger scale production you don't want to condense the acetone out first, but instead quench the hot gases with a liquid
make of acetic acid, acetic anhydride, and acetone. Acetone condensation and recovery is done after that stage.
As I said several posts ago, my Farsi is limited to being able to ask directions for places to get food. It will not suffice in translating the
material in this thread.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Mr not important really thanks
This is very good that you know a simple Farsi SENTENCE(i know members that believe farsi is arabi and they dont know what is farsi!) 
Mr not important i understand english No need for farsi.
you say true but you should accept that your two patent is very professional and this is difficult to underestant fast and complete.
your suggestion is very interesting for me.
according to your suggestion I realized this image
Good idea?
[Edited on 3-12-2007 by Fashist]
Attachment: Untitled-1.zip (24kB)
This file has been downloaded 769 times
|
|
|
WizardX
Hazard to Self
 
Posts: 61
Registered: 11-8-2005
Location: wizardx.4shared.com
Member Is Offline
Mood: wizardx.suddenlaunch3.com
|
|
| Quote: | Originally posted by not_important
Very true, but if you had read the thread you would have seen that it was discussed early on, and is not suitable for the application in question.
Too small of a production rate, the filament requires frequent cleaning, and the apparatus is fragile.
|
The apparatus (above link) is not a ketene lamp.
It is a glass combustion tube filled with broken porcelain, and fourteen of the twenty burners of the combustion furnace are lighted (Note 4) and
tiles are placed over the lighted burners, which finally must be adjusted to yield a maximum temperature. The first two and last four burners are
unused.
Ketene can be generated conveniently by pyrolysis of acetone in a hot tube or over a hot wire in a "ketene lamp," or by pyrolysis of diketene in a hot
tube, or by pyrolysis of acetic anhydride. Other methods of preparation have been summarized. It has been shown that diketene cracks quite cleanly to
ketene , although some allene and carbon dioxide are formed at the same time.
The most convenient procedures for the preparation of ketene are the present one and the pyrolyses of acetone or acetic ahnydride. The acetone
procedure gives ketene at a relatively fast rate (0.45 mole per hour), but it takes considerable adjustment to get optimum conditions, and trouble is
sometimes caused by the wire getting coated with carbon. Furthermore, because the efficiency of a given wire coil varies with time, passing through a
maximum, frequent calibration of the apparatus is necessary. The acetic anhydride method is even faster (1.31 moles per hour) and uses a readily
available chemical. It appears to be the method of choice at this time. The diketene procedure described here is relatively simple and reliable;
however, it is relatively slow (0.2 mole per hour) and requires a somewhat less readily available starting material.
http://www.orgsyn.org/orgsyn/prep.asp?prep=CV5P0679
Albert Einstein - \"Great ideas often receive violent opposition from mediocre minds.\"
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
No idea For My Design?
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
From what I can tell (nothing is labeled) it seems like your design is very similar to the one on orgsyn. What's your reasoning for using 3
combustion tubes? Did I miss something upthread or was this your idea?
I suppose it would increase your surface area in the furnace which would be beneficial if your flow rate was high enough. Whether or not this would
be better than a single large tube is beyond me. Seems like it could be though.
I thought I would take a stab at his question since no one else was.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Mr MajicJigPipe Thanks of your Idea
I send Another picture before.the description of this Picture is similar to that Picture(oldest)
Acording to Mr not important advise i increase surface(for better yield)
the DISTANCE between condenser and exit tube is smaller.
But there is one problem:
I have problem with steel cover for copper tube.(may this work is impossible for copper tube)i should find another cover.
I am still waiting for your idea.
thx
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
OK, problems with your design
The single tube from the boiler needs to be larger, it should have at least half the cross-sectional area as the sum of the cross-section areas of the
heated tubes. Same applies to the single tube collecting the gases from the heated tubes.
Using tall cylinders for absorbing ketene the way you've drawn it will not work well, "Ketene Production..." tells you why right on the 1st page and
also gives a better working alternative; I've said this before.
Why is pounding copper pipe through the center of steel pipe impossible? Can you not find steel pipe with an inside diameter very close to the
outside diameter of the copper?
--------------------------------------------------
Sorry, WizardX , OrgSyn was offline when I clicked the link and I assumed it was the ketene lamp one. The fragility and desired production rate
arguments still hold; the OS example does less that a quarter liter of acetone an hour, while the target is at least five times that.
For scaled up production several heated tubes are needed because using one large tube results in poor heat control.
The chapter on ketenes in Organic Reactions vol 3 has a table of literature reports on making ketene, and I've read other such surveys. In
general tubes with packing give lower conversions than plain tubes, there is one report (Rice, Greenberg, Waters, and Vollrath, J. Am. Chem. Soc, 56,
1760 (1934)) of good yields with a packed tube. Rather open packing with high thermal conductivity appears to work as well as plain heated tubes, it
has been used with larger diameter tubing but is much more difficult to cobble together ones self.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Thanks Mr not_important
May send us (Rice, Greenberg, Waters, and Vollrath, J. Am. Chem. Soc, 56, 1760 (1934)).
Using cool tall cylinders will captive ketene and Probably all of ketene will React with acetic acid.this isnt true?(there is effluence part end of
tube inside the cylinders)
I am still waiting for your advise.
[Edited on 4-12-2007 by Fashist]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
From the first page of "Ketene Production and Utilization Experimental Study" | Quote: | | It was found, for instance, that when the ketene-containing gases (freed from most of the acetone) were passed into water through a sintered glass
bubbler, considerable ketene escaped absorption. This was even the case when two such bubblers were used in series. The sintered glass disks
were an inch in diameter and furnished a very fine spray of bubbles. Aside from the inefficiency of this type of absorption apparatus, additional
objection was found in its great resistance to gas flow; the rate at which acetone could be put through the pyrolysis chamber was thus decreased.
|
They were using bubblers similar to the attached image. The combination of the flow resistance of the fritted glass and
the weight of the liquid in a tall container will cause a lot of back pressure, increasing the likelyhood of actone and ketene leaking out of joints
in the apparatus.
This is why they and other larger scale production examples use absorption towers resembling fractionating columns for distillatiom, in the ketene
plant project the quencher/absorber is a fractionating column, with acetone and the non-condensible gases leaving as vapour.
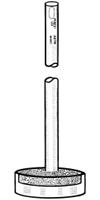
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Thanks Mr not important
You say Complete True
I Think the absorption towers use for industrial scale(not 5-10lit anhydrid per day!)
your Point about "the weight of the liquid in a tall container " is true but I think this is
OBVIOUS for above 10 lit container.(my cylinders is 2lit)
The absorption tower is very UNINTELLIGIBLE and very Expensive for making.
i saw someplace that german army in wwii used of similar system(without absorption tower.like my system but ketene react with acetic in SQUARE
container not CYLINDER)
Wat is your suggestion (replace for tall container?ballon?..)
May you explain this bubblers more?i think there is variant type of bubbler for buy here.
I think if you explain the complete(with detail.like my picture) system for making 5-10lit anhydrid then my question will end(i know you are tired of
my question)
[Edited on 4-12-2007 by Fashist]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
A carrier gas is very nice to surmount the back pressure problem. I can't recall if we used nitrogen or not for our carrier for the acetone vapour.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
What about introducing the ketene into the boiler of a refluxing packed fractionating column of acetic acid?
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Mr Fleaker very nice suggestion(Thanks)
What part we should insert nitrogen or argon?
This work has no effect on ketene in heater tube?
If this suggestion work well then large amount of argon or nitrogen needed.
[Edited on 5-12-2007 by Fashist]
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Today I Found many patent for making anhydrid(using co+methyl acetate under pressure)
But these patent use of very expensive componet.
Anybody has better patent?
thx
Attachment: 4544511.pdf (420kB)
This file has been downloaded 940 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
If you think a proper absorption tower is expensive, try pricing what's needed for for that process; 5 to 20 times atmospheric pressure, 150 to 200 C,
an acidic environment containing carbon monoxide and iodides. Where are you going to get the high purity carbon monoxide needed? That's an entire
plant in itself. If you think an absorption tower is "unintelligible" wait until you see what a carbonylation process is like. And then there is
catalyst recovery to deal with.
This is what I believe is the most recent significant version of the general process
http://www.patentstorm.us/patents/5900504-description.html
Processes used in war time generally are not too concerned with being economical, focusing on using readily available feedstock and being quick to
construct.
To overcome backpressure you need to increase the pressure on the side feeding into the absorption hardware, that is the boiler and hot tube section.
It doesn't matter if you generate the pressure by injecting an inert gas or by running the boiler a little hotter. What's important is that leaks
become a bigger problem.
For someone who says it is "impossible" for them to drive one pipe through the inside of another, you sure are unconcerned about putting together a
system that runs under pressure, even the relatively low pressure to overcome absorber resistance.
------------------------
The goal is to make 5-10 liters anhydride per day.
The systems with the highest overall yield run at low per-pass conversion rates, 5 to 20 percent of the acetone reaction in a pass, the rest is
recirculated. Higher conversion rates result in the formation of products besides ketene, reducing the
overall yield and creating more byproducts.
10 percent conversion per pass means 50 - 100 liters equivalent of acetone,
20 percent is still 25 to 50 liters of acetone boiled off.
With recycling of the acetone the amount used is much less, but you still boil enough to be equivalent to much more acetone without recycling.
Many of the example setups boil off a quarter liter or so per hour, some I've seen can do a liter an hour. The stated goal is roughly 25 to 100 times
greater than that of the lab benchtop models.
[Edited on 5-12-2007 by not_important]
Attachment: routes-to-acetic-anhydride-abs.pdf (220kB)
This file has been downloaded 2449 times
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
| Quote: | Originally posted by not_important
For someone who says it is "impossible" for them to drive one pipe through the inside of another, you sure are unconcerned about putting together a
system that runs under pressure, even the relatively low pressure to overcome absorber resistance.
[Edited on 5-12-2007 by not_important] |
Doesnt Matter. 
Mr not important i think 1-2lit anhydrid per day is more SUITABLE for our.(less problem)
Again we will have Problem if we want 1-2 lit anhydrid per day?
Mr not important Thank you.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Mr not important
There is other way for making anhydrid in your article
Oxidation of acetaldehyde using cobalt salt.
May explain more this Method?
I didnt say that this work(insert copper tube in steel tube) is impossible for me.i said this work(cover copper tube with steel.Not steel tube.this
work isnt USUAL here.)
I think finding better way for making anhydrid has no Problem and isnt sin.
Thanks
[Edited on 6-12-2007 by Fashist]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Unless you're making acetaldehyde from cheap, non-taxed ethanol, it won't be economical. Acetaldehyde has problems of its own (i.e. vapour pressure,
toxicity). Might as well buy Ac2O ready made.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
"Might as well buy Ac2O ready made. "? But the whole reason for this thread is that, in many countries, Ac2O CANNOT be "bought ready made", or at
least not without bring oneself to the attention of the Pigs, because of its being a very useful reagent not only for general acetylations (most often
to protect reactive -OH and other groups during other reactions), but also in drug synthesis, e.g. making heroin by acetylation of other opioids.
I am frankly sick of the Pigs telling us what drugs we can and cannot take, and scaring parliaments and congresses by means of lies (supposedly backed
up by so-called "experts" who are in fact in the pay of alcohol and tobacco interests, who probably also bribe the Pigs) into passing laws to this
effect. If drugs were legal, it would completely destroy the lucrative black-market for them, and thereby make them less easily obtainable, not more
easily obtainable.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The oxidation of acetaldehyde by air or oxygen using a Mn or Cu-Co catalyst takes place at moderate temperatures and pressures, distinctly lower than
the carbonylation process. It does produce and decompose peracetic acid, unless considerable care is take interesting explosions can result. The
plant requirements are more demanding that the ketene methods.
And as already said, it takes cheap acetaldehyde to be practical, and acetaldyhde is difficult to ship and work with.
If all you want is a liter a day, then a single tube ketene generator will work, the absorption issue still exists.
| Quote: | | I think finding better way for making anhydrid has no Problem and isnt sin. |
I've no idea what you meant by that. If you think you'll find a better acetic anhydride production method, you're not going to find it floating about
here. There's been considerable talk on it, there's several threads full of it, including the digging Sauron did here. If there were a simpler good
method it's doubtful it would have gone unused industrially; most of the information has been given to you.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
| Quote: | Originally posted by not_important
If all you want is a liter a day, then a single tube ketene generator will work, the absorption issue still exists.
|
Thanks Mr not important
I said before that i need 5-10 lit per day but i think this is very easy to use 5 apart system(each system make 1 lit per day)because if we want 5-10
in one system then probably we need great professor for invent and operate this system.
as you know most important country cut it trade with iran(like germany,france,...) and many chemical not found here.
the price of all chemical componet increased.(for example
1lit acetyle chloride is 120$) this situation is same for our petrochemical componet for ex the price of acetic acid increaseed.(before .5$ per
lit.now 3$per lit if you find)
here we have bad situation.
Mr johnww thanks.
i should say that law for drug changed here.
if you have 20-30gram heroin police can kill you without any TRIBUNAL.they can kill you there without any Time.
I dont want to make drug because i believe the price of this drug provide with life of youg people.
just i want anhydrid for glue.no more.
soon i must delete all of my posts(i hope you underestant why)
thanks
[Edited on 7-12-2007 by Fashist]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Actually the 5 separate systems will have the same degree of complexity with the exception of the manifolds. The absorption step remains the same,
both for efficiency (cost) and safety issues. Acetic anhydride was not the typical target of laboratory ketene generators, it is for industrial
applications so those are the people you need to look to for information. And they all used packed towers or tall empty towers for their combined
quench-absorption step.
Mostly ignored in this discussion is what purification of the acetic anhydride will be needed. In the industrial style continuous version you get
three product stream - an acetone one to send back to the boilers, an acetic acid rich one containing acetic anhydride and acetone to send to the
quench-absorption unit, and the acetic anhydride product stream. In a small batch system you propose you have to distill the condensed acetone, add
the still bottoms to the acetic product, distill that to get the acetone rich cut, the acetic acid rich cut, and the acetic anhydride cut. Two
distince operations, one with three steps.
The overall effort to operate will be greater, because you have 5 boilers, tube furnaces, and absorption systems to watch.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
The Reaction between ketene and acetic acid is CALEFACTORY or need heat?(accoring to loshatoliye law)
thx
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
This industrial process apparently does it at 80*C.
Although, I have to admit, they do it very differently than a home chemist would.
Attachment: Project00.1.pdf (73kB)
This file has been downloaded 2261 times
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Already posted back on 30-11-2007 at 07:52, and I've refered to it several times since.
| Quote: | Originally posted by MagicJigPipe
This industrial process apparently does it at 80*C.
Attachment: Project00.1.pdf (72.55 KiB)
|
|
|
|
| Pages:
1
..
9
10
11
12
13
14 |