| Pages:
1
2 |
MattEx
Harmless

Posts: 12
Registered: 30-5-2005
Location: Auckland, New Zealand
Member Is Offline
Mood: No Mood
|
|
Pyridine hydrogenation - methods?
Okay - so the idea is that I want to hydrogenate 2-bromopyridine to 2-bromopiperidine. 2-BrPy is MUCH cheaper than 2-BrPpd is the reason.
Ideally i'd like to be able to do this with Raney nickel. H2 or transfer hydrogenation. All and any information relating to this would be highly
appreciated  ! I've never done any hydrogenations, nor studued chemistry to the
level of performing these (i'm at University studying Chemistry at the moment, just not at that level yet). ! I've never done any hydrogenations, nor studued chemistry to the
level of performing these (i'm at University studying Chemistry at the moment, just not at that level yet).
I've searched for information on doing this but haven't really found anything conclusive - found exotic methods for enantiomer-specific syntheses, but
nothing on just plain & simple hydrogenation.
[Edited on by MattEx]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Go to the literature. It isn't very likely anyone will have this in their head.
Have you looked in Merck Index? Org.Syn.? JACS? JOC? or even Google?
Well, Merck negative, Org.Syn negative except for a prep of 2-bromopyridine from 2-aminopyridine by diazotization. JACS negative. One solitary article
in JOC which I will now go request.
Aldrich does not sell 2-bromopiperidine, nor does Acros.
This stuff seems rather obscure.
I now have the JOC article and am reading it. Keep your fingers crossed.
Alas, not pertinent, see for yourself.
I suggest you try the specialist literature on pyridines and piperidines like Saul Patai's series, or Advances in Heterocyclic Chemistry.
As to reduction of bromopyridine, the problem might be lability of the Br. Pyridine per se is easily reduced to piperidine at atmosphere by Na in
ethanol. But in case of a bromopyridine I suspect the Na would abstract the Br.
[Edited on 20-3-2007 by Sauron]
Attachment: jo00244a020[1].pdf (677kB)
This file has been downloaded 1177 times
|
|
|
solo
International Hazard
    
Posts: 3966
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Brief guide to hydrogenation: hydrogenation is a class of chemical reactions in which the net result is an addition of hydrogen. The usual targets of
hydrogenation are unsaturated organic compounds, such as alkenes, alkynes, ketones, nitriles, and imines. Most hydrogenations involve the direct
addition of diatomic hydrogen under pressure in the presence of catalysts. Some hydrogenation involve the indirect addition of hydrogen, these are
called transfer hydrogenation. The reverse reaction, removal of hydrogen, is called hydrogenation.
The classical example of a hydrogenation is the addition of hydrogen on unsaturated bonds between carbon atoms, converting alkenes to alkanes.
hydrogenation is different from the related protonation because in that process just one hydrogen atom is added and not two.
.........source,
http://www.afwtech.com/tech/hydrogenation.html
Also read Rylander's book on Hydrogenation, which can be found on the net.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MattEx
Okay - so the idea is that I want to hydrogenate 2-bromopyridine to 2-bromopiperidine. 2-BrPy is MUCH cheaper than 2-BrPpd is the reason.
|
You can not hydrogenate 2-bromopyridine to 2-bromopiperidine due to obvious reasons. The starting material would have to be piperidine. Besides
2-bromopiperidine is a non-existant compound as such (even its two CA entries lead only to a wrongly abstracted paper and patent). You probably mean
2,3,4,5-tetrahydropyridinium bromide (iminium form) or its tautomere 1,2,3,4-tetrahydropyridinium bromide (enaminium form) to which it spontaneously
transforms as soon as it is formed.
There are only two suppliers in the world claiming to sell this 2-bromopiperidine stuff and both look pretty obscure to me so I would not count too
much on them.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As I already reported, it certainly is the case that this compound is almost completely absent from the literature and that does suggest that maybe it
does not exist.
One last place to look is the ARK group website (Center for Heterocyclic Chemistry, UFL, Alan Katritzshy's group) as they have a large collection of
rare heterocyclic derivatives particularly pyridine and piperidine derivs and they sell them although they are excruciatingly expensive. If the ARK
mob have not prepared 2-bromopiperidine then maybe it's a very long shot.
The corresponding pyridine is available. So you could certainly try to develop a method for selective nuclear hydrogenation to which the 2-bromo
moiety would not be labile. Good luck!
@Nicodem, I'd like to hear an explication of the theoretical reasons why this compound can't exist. Why for example can't piperidine be brominated? I
know why pyridine can't easily be halogenated but, that does not apply to the saturated ring. Probably a messy mixture of isomers but that's what we
have HPLC for.
4-bromopiperidine is commercial and I would be surprised if 3-bromopiperidine were not available as well or preparable from the known
3-hydroxypiperidine which is an important pharm precursor (Ditran).
[Edited on 21-3-2007 by Sauron]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Plain pyridine can be hydrogenated to piperidine by electrolysis of its aqueous solution to which at least 4 mol sulfuric acid per mol pyridine has
been added, using lead electrodes. You don't even need a diaphragma.
However, you have to make sure that no pyridine remains and all gets hydrogenated, because those two cant be separated efficiently by distillation.
You could separate them by acylation with e.g. benzoyl chloride, which will tie up the piperidine as the high-boiling N-Benzoylpiperidine and leave
the pyridine untouched. The benzoylpiperidine can then be hydrolysed with acid or base.
With bromopyridine, its going to become diffficult though. I know of no hydrogenation method that would leave the bromine intact.
Also the bromopiperidine, if it exists in the first place, will be unstable because the Br atom will like to alkylate the N atom.
2-Bromoethylamine is unstable for the same reason.
Or it will eliminate HBr to form the tetrahydropyridinium bromide, as Nicodem said.
[Edited on 21-3-2007 by garage chemist]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, always better to explain these things even when they are "obvious" to some, when there are less experienced people in the audience.
|
|
|
MattEx
Harmless

Posts: 12
Registered: 30-5-2005
Location: Auckland, New Zealand
Member Is Offline
Mood: No Mood
|
|
Nah nah, 2-Br-piperidine IS commercially avaliable, just very expensive - trust me, I got a quote on it (and from a reputable company, nothing sketchy
about it!) .
I didn't find information on hydrogenating 2-Br-pyridine to the piperidine, but I did find information on hydrogenating 2-benzylpyridine to
2-benzylpiperidine - which was my target product anyway (intended on making through Grignard reaction).
So, I should just be able to make 2-Bz-Pyridine, then hydrogenate that.
Stated procedure was Pd/C, room temp, 30 psi for 2 hours, stated ~95% yield.
Worth a try, I think. If I end up doing it I shall post my results...
First off, I need some hydrogenation equipment!
[Edited on by MattEx]
[Edited on by MattEx]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MattEx
Nah nah, 2-Br-piperidine IS commercially avaliable, just very expensive - trust me, I got a quote on it.
|
Alpha-haloamines do not exist unless the amine group is quarternized (as in the famous Selectfluor®, for example) or nitrogen's lone electron pairs
made unavailable by resonance (as in N-chloromethylphthalimide, for example). I'm sorry to say but you got suckered by those suppliers. There is not a
single mention of 2-bromopiperidine in the literature and the suppliers you contacted were probably trying to sell you 2,3,4,5-tetrahydropyridinium
bromide which is formally equivalent to 2-bromopiperidine (thus you would not even be able to file a reclamation). Anyway, this is obviously
irrelevant since you would need 2,3,4,5-tetrahydropyridine for preparing 2-benzylpiperidine if using the benzyl Grignard reagent and not something
like "2-bromopiperidine" which (if existed) would only quench the Grignard. Next time make it clear from the beginning what your post is about so
people do not waste time searching the answer which would be irrelevant for your purpose anyway!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
NeonCortex
Harmless

Posts: 17
Registered: 4-2-2007
Member Is Offline
Mood: No Mood
|
|
Buy the substituted pyridine and hydrogenate over platinum oxide. If you have access to hydrogenation apparatus, this is probably the easiest route.
And the simple substituted pyridines we're talking about aren't very expensive.
Lighting up your cortex since birth
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@nicodem, I wanto know who the "reputable supplier is.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
pyridine reductions
I think the product of commerce might be a tetrahydropyridine @Nicodem (a little harsh but perhaps understandably). Doing a search for
tetrahydropyridines I found an excellant article shared by Solo on the synthesis of such. I seem to recall a post suggesting that pyridine could be
reduced with Na-borohydride but it was short on details .. anyone know more about using hydrides, exchange reactions, electrolytic methods?
Anyway, I have been guilty of vagueness too and it just doesn't help anyone least of all you.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There are two suppliers who registered this "2-bromopiperidine" item at ChemExper, namely Bosche Scientific, LLC (www.boschesci.com) and Shanghai PI Chemicals Ltd (www.pipharm.com) – any free publicity is purely unintentional and I never claimed they were reputable. Not even beta-bromo amines are stabile
enough to be on sale, only their protected/protonated versions are (as for example 2-bromoethylamine hydrobromide etc.) so it is quite obvious that
the so much less stabile alpha-bromo amines would be unattainable.
2,3,4,5-tetrahydropyridine with its tautomere can be easily prepared by the elimination of HBr from 1-bromopiperidine by the use of a base (t-BuOK and
similar) while 1-bromopiperidine is prepared by N-brominating piperidine.
Like I said 2,3,4,5-tetrahydropyridinium bromide has some CA entries, but the 2-bromopiperidine has only two erroneous entries so its CAS number
should be considered bogus. Those two companies could be selling 2,3,4,5-tetrahydropyridinium bromide under the name&structure of
2-bromopiperidine and since these two compounds are in dynamic equilibrium none could accuse them of bullshiting the consumers. In such a case the
consumer would be bullshiting himself - thus a purely normal trade relation in these days of consumerism.
NaBH4 only reduces quarternary pyridinum salts to their 1-alkyl-1,2,3,6-tetrahydropyridine counterparts (which are of no use to the originator of the
thread).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
MattEx
Harmless

Posts: 12
Registered: 30-5-2005
Location: Auckland, New Zealand
Member Is Offline
Mood: No Mood
|
|
Sorry - I wasn't aware of the problems you've now discussed. Now I do.
You said: "Anyway, this is obviously irrelevant since you would need 2,3,4,5-tetrahydropyridine for preparing 2-benzylpiperidine if using the
benzyl Grignard reagent and not something like "2-bromopiperidine" which (if existed) would only quench the Grignard."
Why is this? I would've thought that (had it existed/was stable) that you'd get a standard R-MgBr + Br-R' coupling reaction going on, to form R-R' ?
Or are you implying that the piperidine N would participate instead? I don't catch your drift. Also, how would a Grignard reaction proceed through a
doublebond? None of my notes describe the method you describe.
Anyway i've found a nice paper that nicely describes what I want to do anyway, so no problem - if anyone is interested then here it is:
Convenient, Benign and Scalable Synthesis of 2- and 4-Substituted Benzylpiperidines
B Agai, A Proszenyak, G Tarkanyi, L Vida, F Faigl - European Journal of Organic Chemistry, 2004
[Edited on by MattEx]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MattEx
You said: "Anyway, this is obviously irrelevant since you would need 2,3,4,5-tetrahydropyridine for preparing 2-benzylpiperidine if using the
benzyl Grignard reagent and not something like "2-bromopiperidine" which (if existed) would only quench the Grignard."
Why is this? I would've thought that (had it existed/was stable) that you'd get a standard R-MgBr + Br-R' coupling reaction going on, to form R-R' ?
Or are you implying that the piperidine N would participate instead? I don't catch your drift. Also, how would a Grignard reaction proceed through a
doublebond? None of my notes describe the method you describe. |
I really think you should read more on the Grignard reactions. There simply is no such thing as "a standard R-MgBr + Br-R' coupling reaction". Just the opposite of what you
believe, a standard Grignard reaction is a nucleophilic addition over an electrophilic carbon-heteroatom double bond.
Grignard regents can act as strong bases and get immediately quenched by acids and since a hypothetical 2-bromopiperidine is just a lesser equilibrium
form of 2,3,4,5-tetrahydropyridinium bromide which is an acid (pKa about 8) the reagent would get quenched immediately upon contact (but actually just
about any N-unsubstituted piperidine quenches the Grignard reagents due to the somewhat acidic N-H bond having the pKa a bit less than 36).
Grignard reactions reagents slowly react only with very electrophilic alkyl halides and only with those that have no beta-hydrogens on sp3 hybridized
carbons or heteroatoms (otherwise E2 elimination is preferred over coupling). Typical examples of alkyl halides that react with Grignard reagents are
benzyl and allyl halides (this is why the preparation of benzyl Grignard reagents needs to be carried out carefully – the formed BnMgBr can couple
with unreacted BnBr already at diethyl ether reflux temperature!). Other, that is "normal", alkyl halides do not react with Grignard reagents. If they
do react, they instead rather give elimination side products under harsh enough conditions. However, even such unreactive halides like aryl halides,
can be coupled with Grignard reagents under the conditions of the so called Kumada coupling where a transition metal catalyst (usually a Ni, Cu or Fe
complex like (Ph3P)2NiCl2) is used to induce a mechanism including an organometallic intermediate (note that this is no more a normal
nucleophile+electrophile coupling like a true Grignard reaction!).
In short, Gringard reagents are nucleophilic enough to add on carbon-heteroatom multiple bonds and this is their only general reaction, all the rest
is either less common or special. Among such electrophilic groups that can add a Grignard reagent is also the imine group. Hence the formal addition
of a Grignard reagent on 3,4,5,6-tetrahydropyridine gives 2-benzylpiperidine (after neutralization). I do not know how efficient such a reaction is
(if at all), but considering that 2-benzylpyridine costs only 28Eur/100ml, the reduction is full proof, ridiculously simple and high yielding… well,
I have no doubts which one I would choose.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
| Quote: | | NaBH4 only reduces quarternary pyridinum salts to their 1-alkyl-1,2,3,6-tetrahydropyridine counterparts |
Could you please expand this just a little? It may be of no use to the original poster but I have a passing interest in these. I had read this in an
old, old thread and, as I said, there wasn't a lot of information. Google and Org Syn haven't been helpful and Beilsteins is a trip out of town.
Thanks in advance!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
| Quote: | | NaBH4 only reduces quarternary pyridinum salts to their 1-alkyl-1,2,3,6-tetrahydropyridine counterparts |
Could you please expand this just a little? |
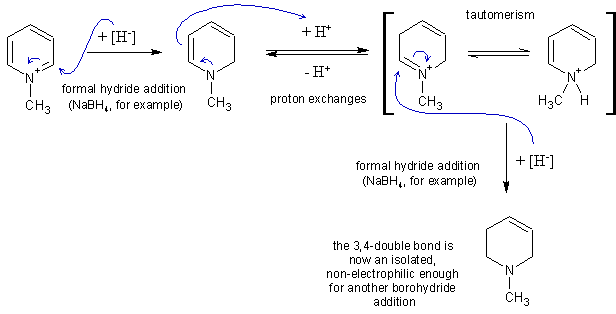
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
janmi
Harmless

Posts: 7
Registered: 9-10-2013
Member Is Offline
Mood: reagent
|
|
Quote: Originally posted by Nicodem  | There are two suppliers who registered this "2-bromopiperidine" item at ChemExper, namely Bosche Scientific, LLC (www.boschesci.com) and Shanghai PI Chemicals Ltd (www.pipharm.com) – any free publicity is purely unintentional and I never claimed they were reputable. Not even beta-bromo amines are stabile
enough to be on sale, only their protected/protonated versions are (as for example 2-bromoethylamine hydrobromide etc.) so it is quite obvious that
the so much less stabile alpha-bromo amines would be unattainable.
2,3,4,5-tetrahydropyridine with its tautomere can be easily prepared by the elimination of HBr from 1-bromopiperidine by the use of a base (t-BuOK and
similar) while 1-bromopiperidine is prepared by N-brominating piperidine.
Like I said 2,3,4,5-tetrahydropyridinium bromide has some CA entries, but the 2-bromopiperidine has only two erroneous entries so its CAS number
should be considered bogus. Those two companies could be selling 2,3,4,5-tetrahydropyridinium bromide under the name&structure of
2-bromopiperidine and since these two compounds are in dynamic equilibrium none could accuse them of bullshiting the consumers. In such a case the
consumer would be bullshiting himself - thus a purely normal trade relation in these days of consumerism.
NaBH4 only reduces quarternary pyridinum salts to their 1-alkyl-1,2,3,6-tetrahydropyridine counterparts (which are of no use to the originator of the
thread). |
Haha, it is so happy to see that my company www.pipharm.com had been quoted here in 2007 in sciencemadness.   
For lab reagents, please just contact us. My email: janmi.zhang@pipharm.com
[Edited on 11-10-2013 by janmi]
[Edited on 11-10-2013 by janmi]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Me being a cynic....Am I seeing a round-about PCP Type-Synthesis?
A usual sequence being the addition of a BromoMagnesium-Benzene Grignard, to a Piperidine-Cyclohexanone Imine/Enamine.
|
|
|
Pickardjr
Harmless

Posts: 45
Registered: 27-5-2013
Member Is Offline
Mood: vapor state
|
|
Just the mention of piperidine gets the usual , typical response. sheezz. For those who cant get or make your own secondary cyclic amine that so
watched, theres always pyrrolidine. Its not regulated . at least not in the U.S.
[Edited on 24-10-2013 by Pickardjr]
|
|
|
palico
Hazard to Self
 
Posts: 58
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
There is a possibility to hydrogenate bromopiridine by lithium in liquid amines such as triethylamine, but I think the problem of N - Br bond cleavege
still exist.
|
|
|
walruslover69
Hazard to Others
  
Posts: 216
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
Would the reduction of pyridine to piperidine be possible with aluminum?
I have seen some posts and literature using sodium metal in ethanol. Is there any reason why aluminum in ethanol with either gallium or mercury
catalyst would not work in the same way?
This paper uses nickel/aluminum allow in NaOH solution, but It seems like this just makes hydrogen and Raney nickel in situ. I'm not sure if it would
work without the nickel or not.
https://pubs.acs.org/doi/10.1021/jo00354a021
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Na/EtOH is a single-electron transfer reduction system. The ionization potential of aluminium (vs. H/H+) is -1.66 V, while for Na/Na+ is -2.71 V. As
such, the reaction does not directly transfer. I don't rule it out, but the reduction of aromatics is not easy.
The original sought compound, 2-bromopiperidine, is incredibly unstable, and obviously cannot be made by any kind of reduction.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I'm interested in the electroreduction garagechemist mentioned, where can I find more info on that?
Reflux condenser?? I barely know her!
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well Hydrogenation is a possibility, Maybe. Depends on the reactivity of that Bromine. Were it Chlorine or Fluorine, ensconced on a Benzene ring, it
might be pretty resistant to hydrogenolysis.
Bromine on a Pyridine or Piperidine ring... I don't know. Might or might not, pose a problem.
Piperidine is kinda famous for poisoning Nobel Metal Catalysts, but there might be a simple fix for that.
Seems I've read, that the problem is caused by Piperidine gumming up the catalyst. The simple fix?
HCl! Yup, employing a suitable solvent and aquouse HCl, renders the piperidine soluble, and frees the catalyst to do its job.
I don't want to seem like a dullard, but why are folks interested in this stuff?
[Edited on 14-5-2021 by zed]
|
|
|
| Pages:
1
2 |